Semaglutide Mitigates Doxorubicin-Induced Hepatic and Renal Damage: Functional, Anti-Inflammatory, and Histopathological Insights
DOI:
https://doi.org/10.54133/ajms.v10i1.2626Keywords:
Anti-inflammatory activity , Doxorubicin , Hepatotoxicity , Nephrotoxicity , SemaglutideAbstract
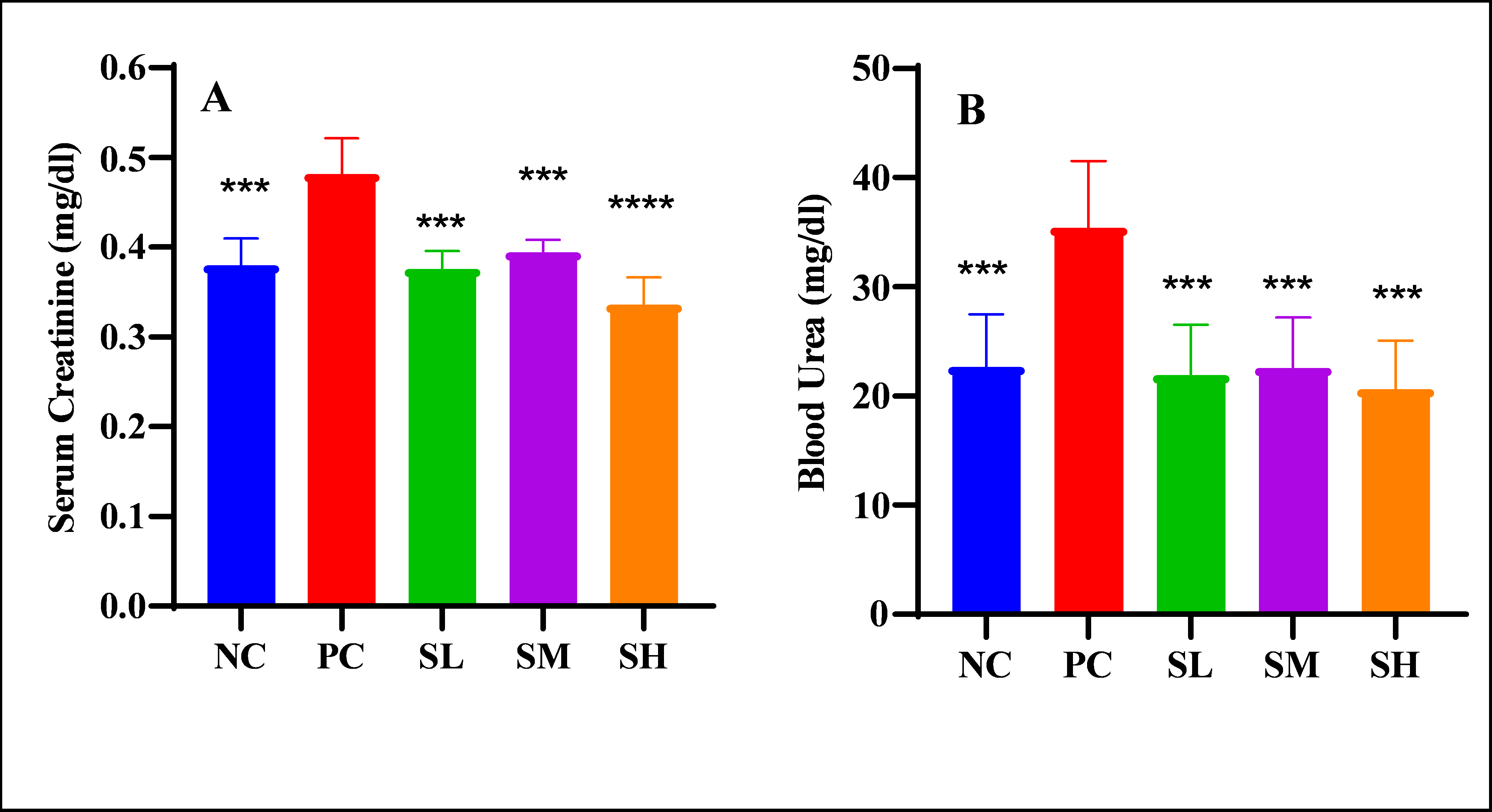
Objective: This research was intended to elucidate the nephroprotective and hepatoprotective effects of semaglutide against doxorubicin-induced toxicity. Methods: Thirty-five female rats were allocated and classified into 5 groups: Negative control: distilled water for the period of seven days. The next group of rats, known as the positive control, was treated with only distilled water in addition to only one dose of doxorubicin (12 mg/kg) on the 7th day. Low dose of semaglutide (SL), moderate dose of semaglutide (SM), and high dose of semaglutide (SH). All semaglutide groups received treatment for the duration of 7 days and a single dose of doxorubicin on the 7th day. On the 8th day, all the animals underwent euthanization, and samples of blood were collected for the purpose of measuring liver enzymes, ADH, urea, creatinine, hs-CRP, TNF-α, IL-10, and CBC. Liver and kidney tissues were submitted for histopathological analysis. Results: Semaglutide groups significantly reduced serum creatinine and blood urea, with a maximum reduction observed in the SH group. The SH group significantly attenuated hs-CRP and TNF-α. All doses of semaglutide significantly elevated the level of IL-10 and ameliorated the granulocyte-to-lymphocyte and platelet-to-lymphocyte ratios compared to the positive control. The microscopical analysis by a histopathologic expert supports the biochemical results as well. Conclusions: Semaglutide possesses hepatic and renoprotective effects via attenuating the biomarkers of liver and kidney damage along with anti-inflammatory activity, with the maximum effects offered by the highest dose of semaglutide.
Downloads
References
Boeno FP, Patel J, Montalvo RN, Lapierre-Nguyen SS, Schreiber CM, Smuder AJ. Effects of exercise preconditioning on doxorubicin-induced liver and kidney toxicity in male and female rats. Int J Mol Sci. 2023;24(12):10222. doi: 10.3390/ijms241210222. DOI: https://doi.org/10.3390/ijms241210222
Prasanna PL, Renu K, Gopalakrishnan AV. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020;250:117599. doi: 10.1016/j.lfs.2020.117599. DOI: https://doi.org/10.1016/j.lfs.2020.117599
Abd-Ellatif RN, Nasef NA, El-Horany HE-S, Emam MN, Younis RL, El Gheit REA, et al. Adrenomedullin mitigates doxorubicin-induced nephrotoxicity in rats: role of oxidative stress, inflammation, apoptosis, and pyroptosis. Int J Mol Sci. 2022;23(23):14570. doi: 10.3390/ijms232314570. DOI: https://doi.org/10.3390/ijms232314570
Smits MM, Van Raalte DH. Safety of semaglutide. Front Endocrinol (Lausanne). 2021;12:645563. doi: 10.3389/fendo.2021.645563. DOI: https://doi.org/10.3389/fendo.2021.645563
Doggrell SA. Sgemaglutide in type 2 diabetes–is it the best glucagon-like peptide 1 receptor agonist (GLP-1R agonist)? Expert Opin Drug Metab Toxicol. 2018;14(3):371-377. doi: 10.1080/17425255.2018.1441286. DOI: https://doi.org/10.1080/17425255.2018.1441286
Reis-Barbosa PH, Marcondes-de-Castro IA, Marinho TS, Aguila MB, Mandarim-de-Lacerda CA. The mTORC1/AMPK pathway plays a role in the beneficial effects of semaglutide (GLP-1 receptor agonist) on the liver of obese mice. Clin Res Hepatol Gastroenterol. 2022;46(6):101922. doi: 10.1016/j.clinre.2022.101922. DOI: https://doi.org/10.1016/j.clinre.2022.101922
Dalbøge LS, Christensen M, Madsen MR, Secher T, Endlich N, Drenic V, et al. Nephroprotective effects of semaglutide as mono- and combination treatment with lisinopril in a mouse model of hypertension-accelerated diabetic kidney Disease. Biomedicines. 2022;10(7):1661. doi: 10.3390/biomedicines10071661. DOI: https://doi.org/10.3390/biomedicines10071661
Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Rønne J, Alanentalo T, Baquero AF, et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight. 2020;5(6): e133429. doi: 10.1172/jci.insight.133429. DOI: https://doi.org/10.1172/jci.insight.133429
Chen X, Zhang Y, Zhu Z, Liu H, Guo H, Xiong C, et al. Protective effect of berberine on doxorubicin‑induced acute hepatorenal toxicity in rats. Mol Med Rep. 2016;13(5):3953-3960. doi: 10.3892/mmr.2016.5017. DOI: https://doi.org/10.3892/mmr.2016.5017
Zhao X, Zhang J, Tong N, Chen Y, Luo Y. Protective effects of berberine on doxorubicin-induced hepatotoxicity in mice. Biol Pharm Bull. 2012;35(5):796-800. doi: 10.1248/bpb.35.796. DOI: https://doi.org/10.1248/bpb.35.796
Nestor JJ, Parkes D, Feigh M, Suschak JJ, Harris MS. Effects of ALT-801, a GLP-1 and glucagon receptor dual agonist, in a translational mouse model of non-alcoholic steatohepatitis. Sci Rep. 2022;12(1):6666. doi: 10.1038/s41598-022-10577-2. DOI: https://doi.org/10.1038/s41598-022-10577-2
Saad SY, Najjar TA, Al-Rikabi AC. The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res. 2001;43(3):211-218. doi: 10.1006/phrs.2000.0769. DOI: https://doi.org/10.1006/phrs.2000.0769
Stark G. Functional consequences of oxidative membrane damage. J Membr Biol. 2005;205:1-16. doi: 10.1007/s00232-005-0753-8. DOI: https://doi.org/10.1007/s00232-005-0753-8
Yang W, Wang J, Shi L, Yu L, Qian Y, Liu Y, et al. Podocyte injury and overexpression of vascular endothelial growth factor and transforming growth factor-beta 1 in adriamycin-induced nephropathy in rats. Cytokine. 2012;59(2):370-376. doi: 10.1016/j.cyto.2012.04.014. DOI: https://doi.org/10.1016/j.cyto.2012.04.014
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844. doi: 10.1056/NEJMoa1607141. DOI: https://doi.org/10.1056/NEJMoa1607141
Mann JF, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839-848. doi: 10.1056/NEJMoa1616011. DOI: https://doi.org/10.1056/NEJMoa1616011
Pavord S, Myers B. Bleeding and thrombotic complications of kidney disease. Blood Rev. 2011;25(6):271-278. doi: 10.1016/j.blre.2011.07.001. DOI: https://doi.org/10.1016/j.blre.2011.07.001
Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms: intermediacy of H2O2-and p53-dependent pathways. J Biol Chem. 2004;279(24):25535-25543. doi: 10.1074/jbc.M400944200. DOI: https://doi.org/10.1074/jbc.M400944200
Kim E, Lim K, Kim K, Bae O, Noh J, Chung S, et al. Doxorubicin‐induced platelet cytotoxicity: a new contributory factor for doxorubicin‐mediated thrombocytopenia. J Thromb Haemost. 2009;7(7):1172-1183. doi: 10.1111/j.1538-7836.2009.03477.x. DOI: https://doi.org/10.1111/j.1538-7836.2009.03477.x
Liu H, Tabuchi T, Takemura A, Kasuga T, Motohashi G, Hiraishi K, et al. The granulocyte/lymphocyte ratio as an independent predictor of tumour growth, metastasis and progression: Its clinical applications. Mol Med Rep. 2008;1(5):699-704. doi: 10.3892/mmr_00000016. DOI: https://doi.org/10.3892/mmr_00000016
Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751-753. doi: 10.1001/jamacardio.2020.1105. DOI: https://doi.org/10.1001/jamacardio.2020.1105
Malavazos AE, Meregalli C, Sorrentino F, Vignati A, Dubini C, Scravaglieri V, et al. Semaglutide therapy decreases epicardial fat inflammation and improves psoriasis severity in patients affected by abdominal obesity and type-2 diabetes. Endocrinol Diabetes Metab Case Rep. 2023;2023(3): 23-0017. doi: 10.1530/EDM-23-0017. DOI: https://doi.org/10.1530/EDM-23-0017
Reppo I, Jakobson M, Volke V. Effects of semaglutide and empagliflozin on inflammatory markers in patients with type 2 diabetes. Int J Mol Sci. 2023;24(6):5714. doi: 10.3390/ijms24065714. DOI: https://doi.org/10.3390/ijms24065714
Newsome P, Francque S, Harrison S, Ratziu V, Van Gaal L, Calanna S, et al. Effect of semaglutide on liver enzymes and markers of inflammation in subjects with type 2 diabetes and/or obesity. Aliment Pharmacol Ther. 2019;50(2):193-203. doi: 10.1111/apt.15316. DOI: https://doi.org/10.1111/apt.15316
Jiang Z, Tan J, Yuan Y, Shen J, Chen Y. Semaglutide ameliorates lipopolysaccharide-induced acute lung injury through inhibiting HDAC5-mediated activation of NF-κB signaling pathway. Hum Exp Toxicol. 2022;41:09603271221125931. doi: 10.1177/09603271221125931. DOI: https://doi.org/10.1177/09603271221125931
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











