Preliminary Evaluation of Tacrolimus Impact on Glycemic Control, Renal Function, and Hematological Inflammatory Markers in Kidney Transplant Recipients
DOI:
https://doi.org/10.54133/ajms.v9i2.2392Keywords:
Glycemic control, Inflammatory markers, Kidney transplantation, Pharmacokinetics, Renal function, TacrolimusAbstract
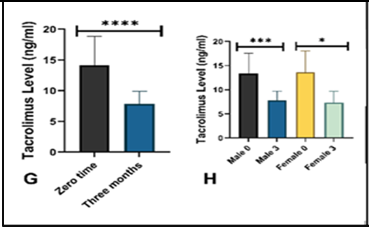
Background: Tacrolimus, a widely used calcineurin inhibitor in kidney transplantation, has a narrow therapeutic window and is associated with metabolic, renal, and hematological effects. Objective: To evaluate the relationship between tacrolimus blood concentrations, glycemic status, kidney function tests, and hematological inflammatory indices in kidney transplant recipients. Methods: This pilot study included 28 kidney allograft recipients, divided into two groups according to tacrolimus trough level (<7.5 ng/mL and >7.5 ng/mL). Fasting blood glucose, serum creatinine, blood urea nitrogen (BUN), and hematological indices, including neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR), were measured at baseline and three months post-transplant. Results: After three months, tacrolimus administration was associated with significant reductions in fasting blood glucose, serum creatinine, and BUN, particularly in female patients. Tacrolimus trough levels declined significantly in both sexes, with a more marked decrease in males. No significant changes were observed in NLR, MLR, PLR, or other hematological indices, and no significant associations were found between tacrolimus concentration groups and the studied parameters. Conclusions: Tacrolimus use over three months post-transplant was linked to improvements in glycemic control and renal function parameters without significant alterations in hematological inflammatory indices. Larger, long-term studies are warranted to confirm these findings and explore potential sex-related differences in tacrolimus pharmacokinetics and clinical effects.
Downloads
References
Zhang L, Guo Y, Ming H, Effects of hemodialysis, peritoneal dialysis, and renal transplantation on the quality of life of patients with end-stage renal disease. Rev Assoc Med Bras. 2020;66(9). doi: 10.1590/1806-9282.66.9.1229. DOI: https://doi.org/10.1590/1806-9282.66.9.1229
Pernin V, Glyda M, Viklický O, Lõhmus A, Wennberg L, Witzke O, et al. Long-term prolonged-release tacrolimus-based immunosuppression in de novo kidney transplant recipients: 5-Y prospective follow-up of patients in the ADVANCE study. Transplant Direct. 2023;9(3):e1432. doi: 10.1097/TXD.0000000000001432. DOI: https://doi.org/10.1097/TXD.0000000000001432
Rostaing L, Jouve T, Terrec F, Malvezzi P, Noble J. Adverse drug events after kidney transplantation. J Pers Med. 2023;13(12):1706. doi: 10.3390/jpm13121706. DOI: https://doi.org/10.3390/jpm13121706
Kolic J, Beet L, Overby P, Cen HH, Panzhinskiy E, Ure DR, et al. Differential effects of voclosporin and tacrolimus on insulin secretion from human islets. Endocrinology. 2020;161(11):bqaa162. doi: 10.1210/endocr/bqaa162. DOI: https://doi.org/10.1210/endocr/bqaa162
Chakkera HA, Mandarino LJ. Calcineurin inhibition and new-onset diabetes mellitus after transplantation. Transplantation. 2013;95(5):647-652. doi: 10.1097/TP.0b013e31826e592e. DOI: https://doi.org/10.1097/TP.0b013e31826e592e
D. Zelle DM, Corpeleijn E, Deinum J, Stolk RP, Gans RO, Navis G, et al. Pancreatic β-cell dysfunction and risk of new-onset diabetes after kidney transplantation. Diabetes Care. 2013;36(7):1926-1932. doi: 10.2337/dc12-1894. DOI: https://doi.org/10.2337/dc12-1894
Iqbal A, Zhou K, Kashyap SR, Lansang MC. Early post-renal transplant hyperglycemia. J Clin Endocrinol Metab. 2022;107(2):549-562. doi: 10.1210/clinem/dgab697. DOI: https://doi.org/10.1210/clinem/dgab697
Hama Salh HJ, Aziz TA, Aziz RS, Mahwi TO. Impact of drug therapy problems on the management of diabetes and hypertension in geriatric patients at Sulaymaniyah City, Iraq. Al-Anbar Med J. 2025;21(3):198–205. DOI: https://doi.org/10.33091/amj.2025.157370.2116
Salh HJH, Aziz TA, Ahmed ZA, Mahwi TO. Association between albuminuria, glycated hemoglobin with comorbidities in type 2 diabetes patients: Experience in Sulaimani City, Iraq. Al-Rafidain J Med Sci. 2024;6(1). doi: 10.54133/ajms.v6i1.380. DOI: https://doi.org/10.54133/ajms.v6i1.380
Maslauskiene R, Vaiciuniene R, Radzeviciene A, Tretjakovs P, Gersone G, Stankevicius E, et al. The influence of tacrolimus exposure and metabolism on the outcomes of kidney transplants. Biomedicines. 2024;12(5):1125. doi: 10.3390/biomedicines12051125. DOI: https://doi.org/10.3390/biomedicines12051125
Nishida S, Ishima T, Iwami D, Nagai R, Aizawa K. Whole blood metabolomic profiling of mice with tacrolimus-induced chronic nephrotoxicity: NAD+ depletion with salvage pathway impairment. Antioxidants (Basel). 2025;14(1):62. doi: 10.3390/antiox14010062. DOI: https://doi.org/10.3390/antiox14010062
Shenoy MT, Manavalan J, A H, K S, Mohanty PK. Tacrolimus concentration/dose ratio: A tool for guiding tacrolimus dosage post-renal transplantation. Cureus. 2024;16(2):e53421. doi: 10.7759/cureus.53421. DOI: https://doi.org/10.7759/cureus.53421
Stefanović NZ, Elickovic-Radovanovic RM, Dankovic KS, Catic-Djordjevic AK, Damnjanovic ID, Mitic BP, et al. Insight into the potential influence of inter-and intra-individual variability of tacrolimus exposure on graft function decline in three-year period following kidney transplantation. Farmacia. 2020;68(6). doi: 10.31925/farmacia.2020.6.10. DOI: https://doi.org/10.31925/farmacia.2020.6.10
Bentata Y. Tacrolimus: 20 years of use in adult kidney transplantation. What we should know about its nephrotoxicity. Artif Organs. 2020;44(2):140-152. doi: 10.1111/aor.13551. DOI: https://doi.org/10.1111/aor.13551
Melissa Flores FM, Rodríguez UV, Cecilia Ruiz MN, Ramirez Ruiz MSFV, Cristina Ramirez AA, WCN24-1557 neutrophil/lymphocyte and platelet/lymphocyte ratio within hemolysis and kidney transplant patients. Kidney Int Rep. 2024;9(4):S124–S125. DOI: https://doi.org/10.1016/j.ekir.2024.02.256
Ercan Emreol H, Büyükkaragöz B, Gönül İI, Bakkaloğlu SA, Fidan K, Söylemezoğlu O, et al. Value of neutrophil-lymphocyte and platelet-lymphocyte ratios in the evaluation of acute rejection and chronic allograft nephropathy in children with kidney transplantation. Exp Clin Transplant. 2022;20(Suppl. 3):129-136. doi: 10.6002/ect.PediatricSymp2022.O41. DOI: https://doi.org/10.6002/ect.PediatricSymp2022.O41
Naranjo M, Agrawal A, Goyal A, Rangaswami J. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict acute cellular rejection in the kidney allograft. Ann Transplant. 2018;23:467-474. doi: 10.12659/AOT.909251. DOI: https://doi.org/10.12659/AOT.909251
Kolonko A, Dwulit T, Skrzypek M, Więcek A. Potential utility of neutrophil-to-lymphocyte, platelet-to-lymphocyte, and neutrophil, lymphocyte, and platelet ratios in differential diagnosis of kidney transplant acute rejection: A retrospective, propensity score matched analysis. Ann Transplant. 2022;27:e937239. doi: 10.12659/AOT.937239. DOI: https://doi.org/10.12659/AOT.937239
Müller MM, Schwaiger E, Kurnikowski A, Haidinger M, Ristl R, Tura A, et al. Glucose metabolism after kidney transplantation: Insulin release and sensitivity with tacrolimus- versus belatacept-based immunosuppression. Am J Kidney Dis. 2021;77(3):462-464. doi: 10.1053/j.ajkd.2020.07.016. DOI: https://doi.org/10.1053/j.ajkd.2020.07.016
Torres A, Hernández D, Moreso F, Serón D, Burgos MD, Pallardó LM, et al. Randomized controlled trial assessing the impact of tacrolimus versus cyclosporine on the incidence of posttransplant diabetes mellitus. Kidney Int Rep. 2018;3(6):1304-1315. doi: 10.1016/j.ekir.2018.07.009. DOI: https://doi.org/10.1016/j.ekir.2018.07.009
Dorantes-Carrilloa LA, Medina-Escobedob M, Cobá-Cantoc YA, Alvarez-Baezac A, Domínguez NM. Glucose homeostasis changes and pancreatic β-cell proliferation after switching to cyclosporin in tacrolimus-induced diabetes mellitus. Nefrologia, 2015;35(3). doi: 10.1016/j.nefroe.2015.06.006. DOI: https://doi.org/10.1016/j.nefroe.2015.06.006
Li Z, Sun F, Zhang Y, Chen H, He N, Chen H, et al. Tacrolimus induces insulin resistance and increases the glucose absorption in the jejunum: A potential mechanism of the diabetogenic effects. PLoS One. 2015;10(11):e0143405. doi: 10.1371/journal.pone.0143405. DOI: https://doi.org/10.1371/journal.pone.0143405
Artz MA, Boots JM, Ligtenberg G, Roodnat JI, Christiaans MH, Vos PF, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. J Am Soc Nephrol. 2003;14(7):1880-1888. doi: 10.1097/01.asn.0000071515.27754.67. DOI: https://doi.org/10.1097/01.ASN.0000071515.27754.67
Kamil HYM, Abdalla AM. Effect of tacrolimus treatment on blood glucose level among renal transplanted Sudanese patients. J Adv Med Dent Sci Res. 2019;7(3):11-15. doi: 10.21276/jamdsr. DOI: https://doi.org/10.21276/jamdsr
Malinowski M, Martus P, Lock JF, Neuhaus P, Stockmann M. Systemic influence of immunosuppressive drugs on small and large bowel transport and barrier function. Transpl Int. 2011;24(2):184-93. doi: 10.1111/j.1432-2277.2010.01167.x. DOI: https://doi.org/10.1111/j.1432-2277.2010.01167.x
Velickovic-Radovanovic R, Mikov M, Catic-Djordjevic A, Stefanovic N, Stojanovic M, Jokanovic M, Cvetkovic T. Tacrolimus as a part of immunosuppressive treatment in kidney transplantation patients: sex differences. Gend Med. 2012;9(6):471-480. doi: 10.1016/j.genm.2012.10.003. DOI: https://doi.org/10.1016/j.genm.2012.10.003
Alghanem SS, Soliman MM, Alibrahim AA, Gheith O, Kenawy AS, Awad A. Monitoring tacrolimus trough concentrations during the first year after kidney transplantation: A national retrospective cohort study. Front Pharmacol. 2020;11:566638. doi: 10.3389/fphar.2020.566638. DOI: https://doi.org/10.3389/fphar.2020.566638
Cheung CY, Chan HW, Liu YL, Chau KF, Li CS. Long-term graft function with tacrolimus and cyclosporine in renal transplantation: paired kidney analysis. Nephrology (Carlton). 2009;14(8):758-763. doi: 10.1111/j.1440-1797.2009.01155.x. DOI: https://doi.org/10.1111/j.1440-1797.2009.01155.x
Lee WC, Lian JD, Wu MJ, Cheng CH, Chen CH, Shu KH. Long-term beneficial effect of tacrolimus conversion on renal transplant recipients. Ren Fail. 2005;27(5):501-506. doi: 10.1080/08860220500198086. DOI: https://doi.org/10.1080/08860220500198086
Shiraishi Y, Amiya E, Hatano M, Katsuki T, Bujo C, Tsuji M, et al. Impact of tacrolimus versus cyclosporin A on renal function during the first year after heart transplant. ESC Heart Fail. 2020;7(4):1842-1849. doi: 10.1002/ehf2.12749. DOI: https://doi.org/10.1002/ehf2.12749
Jurewicz WA. Tacrolimus versus cyclosporin immunosuppression: long-term outcome in renal transplantation. Nephrol Dial Transplant. 2003;18 Suppl 1:i7-11. doi: 10.1093/ndt/gfg1028. DOI: https://doi.org/10.1093/ndt/gfg1028
Yoneda T, Iemura Y, Onishi K, Hori S, Nakai Y, Miyake M, et al. Effect of gender differences on transplant kidney function. Transplant Proc. 2017;49(1):61-64. doi: 10.1016/j.transproceed.2016.10.015. DOI: https://doi.org/10.1016/j.transproceed.2016.10.015
Tornatore KM, Meaney CJ, Attwood K, Brazeau DA, Wilding GE, Consiglio JD, et al. Race and sex associations with tacrolimus pharmacokinetics in stable kidney transplant recipients. Pharmacotherapy. 2022;42(2):94-105. doi: 10.1002/phar.2656. DOI: https://doi.org/10.1002/phar.2656
Velicković-Radovanović R, Mikov M, Paunović G, Djordjević V, Stojanović M, Cvetković T, et al. Gender differences in pharmacokinetics of tacrolimus and their clinical significance in kidney transplant recipients. Gend Med. 2011;8(1):23-31. doi: 10.1016/j.genm.2011.01.003. DOI: https://doi.org/10.1016/j.genm.2011.01.003
Deepalakshmi E, Sowmya K, Sowmiya T, Santhi S. Association of NLR, MLR, PLR, SII, and SIRI with the stages of chronic kidney disease: A cross-sectional study. Int J Med Biochem. 2024;7(3):186–194. doi: 10.14744/ijmb.2024.98150. DOI: https://doi.org/10.14744/ijmb.2024.98150
Stephenson SS, Kravchenko G, Korycka-Błoch R, Kostka T, Sołtysik BK. How immunonutritional markers are associated with age, sex, body mass index and the most common chronic diseases in the hospitalized geriatric population-A cross sectional study. Nutrients. 2024;16(15):2464. doi: 10.3390/nu16152464. DOI: https://doi.org/10.3390/nu16152464
Ismaeel IA, Qadir BH, Ahmed ZA, Hassan OH, Aziz TA. Effects of saroglitazar gel in thermally induced burn in rats. Al-Anbar Med J. 2024;20(2):230–237.
Rasheed RH, Aziz TA. Cardioprotective effects of SAR through attenuating cardiac-specific markers, inflammatory markers, oxidative stress, and anxiety in rats challenged with 5-fluorouracil. J Xenoiot. 2025;15(4):130. doi: 10.3390/jox15040130. DOI: https://doi.org/10.3390/jox15040130
Ali HH, Ahmed ZA, Aziz TA. Effect of telmisartan and quercetin in 5 fluorouracil-Induced renal toxicity in rats. J Inflamm Res. 2022;15:6113-6124. doi: 10.2147/JIR.S389017. DOI: https://doi.org/10.2147/JIR.S389017
Mureșan AV, Russu E, Arbănași EM, Kaller R, Hosu I, Arbănași EM, et al. The predictive value of NLR, MLR, and PLR in the outcome of end-stage kidney disease patients. Biomedicines. 2022;10(6):1272. doi: 10.3390/biomedicines10061272. DOI: https://doi.org/10.3390/biomedicines10061272
Ergin G, Değer M, Köprü B, Derici Ü, Arınsoy T. High neutrophil to lymphocyte ratio predicts acute allograft rejection in kidney transplantation; a retrospective study. Turk J Med Sci. 2019;49(2):525-530. doi: 10.3906/sag-1811-41. DOI: https://doi.org/10.3906/sag-1811-41
Cvetkovic TP, Stefanovic NZ, Velickovic-Radovanovic RM, Paunovic GJ, Djordjevic VM, Stojanovic DR, et al. Gender differences in oxidative and nitrosative stress parameters in kidney transplant patients on tacrolimus-based immunosuppression. Int Urol Nephrol. 2014;46(6):1217-1224. doi: 10.1007/s11255-013-0577-x. DOI: https://doi.org/10.1007/s11255-013-0577-x
Urbanowicz T, Olasińska-Wiśniewska A, Michalak M, Rodzki M, Witkowska A, Straburzyńska-Migaj E, et al. Neutrophil to lymphocyte ratio (NLR) as an easily accessible parameter for monitoring tacrolimus overdose after heart transplantation: Experimental study. Diagnostics (Basel). 2021;12(1):37. doi: 10.3390/diagnostics12010037. DOI: https://doi.org/10.3390/diagnostics12010037
You J, Chen R, Chai Y, Wang X, Xie W, Yang Y, et al. Comparing tacrolimus level monitoring in peripheral blood mononuclear cells and whole blood within one year after kidney transplantation: a single-center, prospective, observational study. Front Pharmacol. 2025;16:1622702. doi: 10.3389/fphar.2025.1622702. DOI: https://doi.org/10.3389/fphar.2025.1622702
MY, Rasheed JI, Hussein MR. Comparison of side effect between cyclosporine and tacrolimus as immunosuppressive therapy in Iraqi kidney transplant recipients. Ann Trop Med Public Health. 2020; 23(13). doi: 10.36295/ASRO.2020.231349. DOI: https://doi.org/10.36295/ASRO.2020.231349
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











