Evaluating CASPASE-9 Gene Expression and Protein Level in Iraqi Patients with β-Thalassemia Major
DOI:
https://doi.org/10.54133/ajms.v9i1.2093الكلمات المفتاحية:
Beta thalassemia major، CASP9 gene، Real Time PCRالملخص
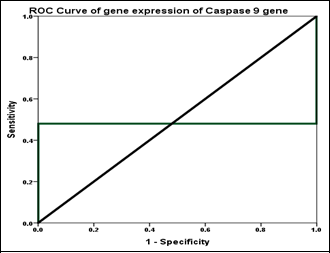
Background: Caspase-9 (CASP9) is a cysteine-dependent, aspartate-specific protease primarily recognized for its role in apoptosis. It functions as an initiator caspase in the intrinsic (mitochondrial) pathway of apoptosis, leading to programmed cell death. Objectives: To assess the gene expression of CASP9 and its protein level in β-thalassemia major (β-TM) patients compared to healthy controls. Methods: The study included 100 participants, 50 of whom had β-TM, whereas the other 50 served as controls. To assess the CASP9 transcript levels, blood samples were collected from each participant, and RNA extraction was performed. cDNA synthesis was carried out, and real-time PCR was utilized for the analysis. Results: No statistically significant difference was observed in the age distribution between the β-thalassemia major (β-TM) patients and the healthy controls (p>0.05), with a mean age of 15.58 and 15.24 years, respectively. The gender was comparable between groups (p>0.05), indicating that the groups were well-matched. The CASP9 gene was significantly overexpressed in β-TM patients, with a fold change of 2.04 compared to 1.0 in controls. Gene expression was quantified using quantitative real-time PCR (qRT-PCR), while protein levels were measured using enzyme-linked immunosorbent assay (ELISA). A positive correlation was observed between CASP9 mRNA expression and its corresponding protein levels (r=0.489, p<0.0001). Conclusions: CASP9 was overexpressed in β-TM patients and was positively correlated with its protein level, indicating that transcriptional upregulation is associated with increased protein expression in β-TM patients.
التنزيلات
المراجع
Hadi RA, Hamid ZA. The frequency of hepatitis C viral infections and its correlation with IL-12 and IL-18 among major thalassemic patients in Baghdad. Egypt J Chem. 2022;65(8):593-600. doi: 10.21608/ejchem.2021.112813.5126. DOI: https://doi.org/10.21608/ejchem.2021.112813.5126
Kadhim AJ, El-Yaseen HD, Jawad AM. The Level of heart-type fatty acid binding protein (H-FABP) as risk marker for cardiac dysfunction among some beta-thalassemia major patients in Baghdad city-Iraq. Pak J Life Soc Sci. 2024;22(1):3699-3706. doi: 10.57239/PJLSS-2024-22.1.00270. DOI: https://doi.org/10.57239/PJLSS-2024-22.1.00270
Iolascon A, Andolfo I, Russo R. Congenital dyserythropoietic anemias. Blood. 2020;136(11):1274-1283. doi: 10.1182/blood.2019000948. DOI: https://doi.org/10.1182/blood.2019000948
Alazzawi AA, Ghaloub AN, Yaaqoob LA. Investigating the antioxidant and apoptosis inducing effects of biologically synthesized silver nanoparticles against lymphoma cells in vitro. Iraqi J Sci. 2023:4390-403. doi: 10.24996/ijs.2023.64.9.9. DOI: https://doi.org/10.24996/ijs.2023.64.9.9
Khalil AW, Altaee MF. Caspase 9 gene expression and caspase 9 protein in chronic myeloid leukemia in Iraq. Iraqi J Agricult Sci. 2022;53(5):994-1002. doi: 10.36103/ijas.v53i5.1613. DOI: https://doi.org/10.36103/ijas.v53i5.1613
Schug ZT, Gonzalvez F, Houtkooper RH, Vaz FM, Gottlieb E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011;18(3):538-548. doi: 10.1038/cdd.2010.135. DOI: https://doi.org/10.1038/cdd.2010.135
Avrutsky MI, Troy CM. Caspase-9: A multimodal therapeutic target with diverse cellular expression in human disease. Front Pharmacol. 2021;12:701301. doi: 10.3389/fphar.2021.701301. DOI: https://doi.org/10.3389/fphar.2021.701301
Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, et al. Caspase-9: structure, mechanisms and clinical application. Oncotarget. 2017;8(14):23996-24008. doi: 10.18632/oncotarget.15098. DOI: https://doi.org/10.18632/oncotarget.15098
Hussar P. Apoptosis regulators bcl-2 and caspase-3. Encyclopedia. 2022;2(4):1624-1636. doi: 10.3390/encyclopedia2040111. DOI: https://doi.org/10.3390/encyclopedia2040111
Chaichompoo P, Nithipongvanitch R, Kheansaard W, Tubsuwan A, Srinoun K, Vadolas J, et al. Increased autophagy leads to decreased apoptosis during β-thalassaemic mouse and patient erythropoiesis. Sci Rep. 2022;12(1):18628. doi: 10.1038/s41598-022-21249-6. DOI: https://doi.org/10.1038/s41598-022-21249-6
Besse K, Maiers M, Confer D, Albrecht M. On modeling human leukocyte antigen-Identical sibling match probability for allogeneic hematopoietic cell transplantation: Estimating the need for an unrelated donor source. Biol Blood Marrow Transplant. 2016;22(3):410-417. doi: 10.1016/j.bbmt.2015.09.012. DOI: https://doi.org/10.1016/j.bbmt.2015.09.012
Shoshan-Barmatz V, Arif T, Shteinfer-Kuzmine A. Apoptotic proteins with non-apoptotic activity: expression and function in cancer. Apoptosis. 2023;28(5-6):730-753. doi: 10.1007/s10495-023-01835-3. DOI: https://doi.org/10.1007/s10495-023-01835-3
Ye L, Wang J, Tan Y, Beyer AI, Xie F, Muench MO, et al. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: An approach for treating sickle cell disease and β-thalassemia. Proc Natl Acad Sci U S A. 2016;113(38):10661-10665. doi: 10.1073/pnas.1612075113. DOI: https://doi.org/10.1073/pnas.1612075113
Fibach E, Dana M. Oxidative stress in β-thalassemia. Mol Diagn Ther. 2019;23(2):245-261. doi: 10.1007/s40291-018-0373-5. DOI: https://doi.org/10.1007/s40291-018-0373-5
Al-Karawi AS, Kadhim AS. Correlation of autoimmune response and immune system components in the progression of IgA nephropathy: A comparative study. Hum Immunol. 2024;85(6):111181. doi: 10.1016/j.humimm.2024.111181. DOI: https://doi.org/10.1016/j.humimm.2024.111181
Kadhim AS, Al-Karawi AS. Correlation between vitamin D3 levels, autoantibodies, and antibody-related diseases in patients with Hashimoto's thyroiditis. Turkish J Immunol. 2024;12(3):92-97. doi: 10.4274/tji.galenos.2024.65982. DOI: https://doi.org/10.4274/tji.galenos.2024.65982
Longo F, Piolatto A, Ferrero GB, Piga A. Ineffective erythropoiesis in β-Thalassaemia: Key steps and therapeutic options by drugs. Int J Mol Sci. 2021;22(13):7229. doi: 10.3390/ijms22137229. DOI: https://doi.org/10.3390/ijms22137229
Lin S, Zheng Y, Chen M, Xu L, Huang H. The interactions between ineffective erythropoiesis and ferroptosis in β-thalassemia. Front Physiol. 2024;15:1346173. doi: 10.3389/fphys.2024.1346173. DOI: https://doi.org/10.3389/fphys.2024.1346173
Elsahookie M, Cheyed S, Dawood A. An overview on mechanism of gene expression regulation. Iraqi J Agricult Sci. 2021;52(2):454-460. doi: 10.36103/ijas.v52i2.1307. DOI: https://doi.org/10.36103/ijas.v52i2.1307
Walter PB, Porter J, Evans P, Kwiatkowski JL, Neufeld EJ, Coates T, et al. Increased leucocyte apoptosis in transfused β-thalassaemia patients. Br J Haematol. 2013;160(3):399-403. doi: 10.1111/bjh.12076. DOI: https://doi.org/10.1111/bjh.12076
Ficarra S, Tellone E, Giardina B, Scatena R, Russo A, Misiti F, et al. Derangement of erythrocytic AE1 in beta-thalassemia by caspase 3: pathogenic mechanisms and implications in red blood cell senescence. J Membr Biol. 2009;228(1):43-49. doi: 10.1007/s00232-009-9157-5. DOI: https://doi.org/10.1007/s00232-009-9157-5
Urbani A, Prosdocimi E, Carrer A, Checchetto V, Szabò I. Mitochondrial ion channels of the inner membrane and their regulation in cell death signaling. Front Cell Dev Biol. 2021;8:620081. doi: 10.3389/fcell.2020.620081. DOI: https://doi.org/10.3389/fcell.2020.620081
Raducka-Jaszul O, Bogusławska DM, Jędruchniewicz N, Sikorski AF. Role of extrinsic apoptotic signaling pathway during definitive erythropoiesis in normal patients and in patients with β-Thalassemia. Int J Mol Sci. 2020;21(9):3325. doi: 10.3390/ijms21093325. DOI: https://doi.org/10.3390/ijms21093325
التنزيلات
منشور
كيفية الاقتباس
إصدار
القسم
الرخصة
الحقوق الفكرية (c) 2025 Al-Rafidain Journal of Medical Sciences

هذا العمل مرخص بموجب Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).










