Effects of Rosuvastatin on the Histopathological and Inflammatory Markers of Hyperlipidemic Rat Kidneys
DOI:
https://doi.org/10.54133/ajms.v9i2.2247Keywords:
Hyperlipidemia, Histopathology, Inflammatory markers, KidneysAbstract
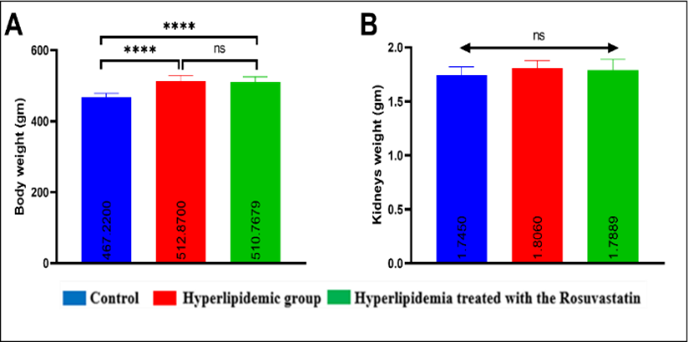
Background: Hyperlipidemia is a significant cause of cardiovascular disease and global death by inducing endothelial dysfunction and narrowing blood vessels. The kidney is the first organ damaged by hyperlipidemia that distracts endothelial capillaries and increases glomerulosclerosis due to decreased renal blood flow. Rosuvastatin has an anti-hyperlipidemic effect and also improves endothelial function and reduces inflammation and oxidative stress. Objectives: The present study aimed to investigate the impact of rosuvastatin on histopathological and inflammatory marker changes induced by hyperlipidemia in male rats. Methods: Forty-six normal male rats were randomly divided into three main groups: the control negative group, ten rats that received a standard diet and water. In the hyperlipidemic group, eighteen rats were fed a high cholesterol (atherogenic) diet (79% standard diet and 21% ghee fat) for seven weeks. The treatment group includes eighteen rats that received a high cholesterol (atherogenic) diet (79% standard diet and 21% ghee fat) for seven weeks and were then treated with rosuvastatin 10 mg/kg/day orally by gastric gavage for 4 weeks. Results: Rosuvastatin significantly decreased the proinflammatory markers (Interleukin-1, Interleukin-6, Tumor Necrosis Factor, and Cystatin C) and the pathological changes in the kidneys (p<0.005) compared with the hyperlipidemic group, while they were significantly increased in the hyperlipidemic group compared to the control group (p<0.005). However, rosuvastatin or hyperlipidemia did not significantly affect the body and the testicular weight. Conclusions: Rosuvastatin modulates the histopathological changes and decreases the proinflammatory cytokine levels in hyperlipidemic male rats.
Downloads
References
Muller CR, Leite APO, Yokota R, Pereira RO, Americo ALV, Nascimento NR, et al. Post weaning exposure to high fat diet induces kidney lipid accumulation and function impairment in adult rats. Front Nutr. 2019;6:60. doi: 10.3389/fnut.2019.00060. DOI: https://doi.org/10.3389/fnut.2019.00060
Bajželj B, Laguzzi F, Röös E. The role of fats in the transition to sustainable diets. Lancet Planet Health. 2021;5(9):e644 e653. doi: 10.1016/S2542 5196(21)00194 7. DOI: https://doi.org/10.1016/S2542-5196(21)00194-7
Aranceta J, Pérez Rodrigo C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: a systematic review. Br J Nutr. 2012;107(S2): S8 S22. doi: 10.1017/S0007114512001210. DOI: https://doi.org/10.1017/S0007114512001444
Salim HM, Kurnia LF, Bintarti TW. The effects of high fat diet on histological changes of kidneys in rats. Biomol Health Sci J. 2018;1(2):109 112. doi: 10.1016/j.bhsj.2018.07.003. DOI: https://doi.org/10.20473/bhsj.v1i2.9675
Wang Y, Jin M, Cheng CK, Li Q. Tubular injury in diabetic kidney disease: molecular mechanisms and potential therapeutic perspectives. Front Endocrinol (Lausanne). 2023;14:1238927. doi: 10.3389/fendo.2023.1238927. DOI: https://doi.org/10.3389/fendo.2023.1238927
Baaten CC, Vondenhoff S, Noels H. Endothelial cell dysfunction and increased cardiovascular risk in patients with chronic kidney disease. Circ Res. 2023;132(8):970 992. doi: 10.1161/CIRCRESAHA.123.321752. DOI: https://doi.org/10.1161/CIRCRESAHA.123.321752
Moorhead JF, El Nahas M, Chan MK, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo interstitial disease. Lancet. 1982;320(8311):1309 1311. doi: 10.1016/S0140 6736(82)91513 6. DOI: https://doi.org/10.1016/S0140-6736(82)91513-6
De Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al. Fatty kidney: emerging role of ectopic lipid in obesity related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417 426. doi: 10.1016/S2213 8587(14)70065 8. DOI: https://doi.org/10.1016/S2213-8587(14)70065-8
Liang X, Ye M, Tao M, Zheng D, Cai R, Zhu Y, et al. The association between dyslipidemia and the incidence of chronic kidney disease in the general Zhejiang population: a retrospective study. BMC Nephrol. 2020;21(1):252. doi: 10.1186/s12882 020 01969 5. DOI: https://doi.org/10.1186/s12882-020-01907-5
Muller CR, Américo ALV, Fiorino P, Evangelista FS. Aerobic exercise training prevents kidney lipid deposition in mice fed a cafeteria diet. Life Sci. 2018;211:140 146. doi: 10.1016/j.lfs.2018.08.012. DOI: https://doi.org/10.1016/j.lfs.2018.09.017
Martins AR, Más S. Lipotoxicity and kidney. Port J Nephrol Hypert. 2015;29(4):306 315. doi: 10.1097/NHN.0000000000000041.
Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21(2):79 85. doi: 10.1016/j.numecd.2010.02.008. DOI: https://doi.org/10.1016/j.numecd.2010.10.002
Takabatake Y, Yamamoto T, Isaka Y. Stagnation of autophagy: A novel mechanism of renal lipotoxicity. Autophagy. 2017;13(4):775 776. doi: 10.1080/15548627.2017.1285147. DOI: https://doi.org/10.1080/15548627.2017.1283084
Abrass CK. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol. 2004;24:46 53. doi: 10.1159/000080268. DOI: https://doi.org/10.1159/000075925
Keane WF. The role of lipids in renal disease: Future challenges. Kidney Int Suppl. 2000;57:S27 31. doi: 10.1046/j.1523 1755.2001.057suppl_57s27.x. DOI: https://doi.org/10.1046/j.1523-1755.2000.07503.x
Zhou Y, Lin S, Zhang L, Li Y. Resveratrol prevents renal lipotoxicity in high fat diet treated mouse model through regulating PPAR α pathway. Mol Cell Biochem. 2016;411:143 150. doi: 10.1007/s11010 015 2534 7.
Hussain A, Kaler J, Ray SD. The Benefits outweigh the risks of treating hypercholesterolemia: The statin dilemma. Cureus. 2023;15(1):e33648. doi: 10.7759/cureus.33648. DOI: https://doi.org/10.7759/cureus.33648
Ma Y, Su Q, Yue C, Zou H, Zhu J, Zhao H, et al. The effect of oxidative stress induced autophagy by cadmium exposure in kidney, liver, and bone damage, and neurotoxicity. Int J Mol Sci. 2022;23(21):13491. doi: 10.3390/ijms232113491. DOI: https://doi.org/10.3390/ijms232113491
Hassan N, El Bassossy HM, Zakaria MNM. Heme oxygenase 1 induction protects against hypertension associated with diabetes: effect on exaggerated vascular contractility. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:217 226. doi: 10.1007/s00210 013 0855 8. DOI: https://doi.org/10.1007/s00210-012-0822-3
Haruhara K, Tsuboi N, Koike K, Fukui A, Miyazaki Y, Kawamura T, et al. Renal histopathological findings in relation to ambulatory blood pressure in chronic kidney disease patients. Hypertens Res. 2015;38(2):116 122. doi: 10.1038/hr.2014.161. DOI: https://doi.org/10.1038/hr.2014.140
Thanh TN, Van PD, Cong TD, Le Minh T, Vu QHN. Assessment of testis histopathological changes and spermatogenesis in male mice exposed to chronic scrotal heat stress. J Anim Behav Biometeorol. 2020;8(3):174–180. doi: 10.31893/jabb.20023. DOI: https://doi.org/10.31893/jabb.20023
Vinué Á, Herrero Cervera A, González Navarro H. Understanding the impact of dietary cholesterol on chronic metabolic diseases through studies in rodent models. Nutrients. 2018;10(7):939. doi: 10.3390/nu10070939. DOI: https://doi.org/10.3390/nu10070939
Kelle BP, Ćesić AK, Čustović S, Ćosović E, Lagumdžija D, Jordamović N, et al. Improvement of a diet-induced model of hyperlipidemia in Wistar rats: Assessment of biochemical parameters, the thickness of the abdominal aorta and liver histology. J King Saud Univ Sci. 2024;36(2):103068. doi: 10.1016/j.jksus.2023.103068. DOI: https://doi.org/10.1016/j.jksus.2023.103068
Sidorova YS, Petrov NA, Markova YM, Kolobanov AI, Zorin SN. The Influence of a High Cholesterol Diet and Forced Training on Lipid Metabolism and Intestinal Microbiota in Male Wistar Rats. Int J Mol Sci. 2024;25(10):5383. doi: 10.3390/ijms25105383. DOI: https://doi.org/10.3390/ijms25105383
Schell M, Chudoba C, Leboucher A, Alfine E, Flore T, Ritter K, et al. Interplay of dietary fatty acids and cholesterol impacts brain mitochondria and insulin action. Nutrients. 2020;12(5):1518. doi: 10.3390/nu12051518. DOI: https://doi.org/10.3390/nu12051518
Tan BL, Norhaizan ME. Effect of high fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. 2019;11(11):2579. doi: 10.3390/nu11112579. DOI: https://doi.org/10.3390/nu11112579
Oosterman JE, Foppen E, van der Spek R, Fliers E, Kalsbeek A, la Fleur SE. Timing of fat and liquid sugar intake alters substrate oxidation and food efficiency in male Wistar rats. Chronobiol Int. 2015;32(2):289–298. doi: 10.3109/07420528.2014.979622. DOI: https://doi.org/10.3109/07420528.2014.971177
Guebre Egziabher F, Alix PM, Koppe L, Pelletier CC, Kalbacher E, Fouque D, et al. Ectopic lipid accumulation: A potential cause for metabolic disturbances and a contributor to the alteration of kidney function. Biochimie. 2013;95(11):1971–1979. doi: 10.1016/j.biochi.2013.04.022. DOI: https://doi.org/10.1016/j.biochi.2013.07.017
Ichimura M, Kawase M, Masuzumi M, Sakaki M, Nagata Y, Tanaka K, et al. High fat and high cholesterol diet rapidly induces non alcoholic steatohepatitis with advanced fibrosis in Sprague–Dawley rats. Hepatol Res. 2015;45(4):458–469. doi: 10.1111/hepr.12317. DOI: https://doi.org/10.1111/hepr.12358
Bortolin RC, Vargas AR, Gasparotto J, Chaves PR, Schnorr CE, Martinello KB, et al. A new animal diet based on human Western diet is a robust diet induced obesity model: comparison to high fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int J Obes (Lond). 2018;42(3):525–534. doi: 10.1038/ijo.2017.227. DOI: https://doi.org/10.1038/ijo.2017.225
Choi Y, Jang S, Choi MS, Ryoo ZY, Park T. Increased expression of FGF1 mediated signaling molecules in adipose tissue of obese mice. J Physiol Biochem. 2016;72:157–167. doi: 10.1007/s13105-016-0494-3. DOI: https://doi.org/10.1007/s13105-016-0468-6
Picklo MJ Sr, Idso J, Seeger DR, Aukema HM, Murphy EJ. Comparative effects of high oleic acid vs high mixed saturated fatty acid obesogenic diets upon PUFA metabolism in mice. Prostaglandins Leukot Essent Fatty Acids. 2017;119:25–37. doi: 10.1016/j.plefa.2017.01.006. DOI: https://doi.org/10.1016/j.plefa.2017.03.001
Gaudette É, Goldman DP, Messali A, Sood N. Do statins reduce the health and health care costs of obesity? Pharmacoeconomics. 2015;33:723–734. doi: 10.1007/s40273-015-0280-2. DOI: https://doi.org/10.1007/s40273-014-0234-y
Wicks SE, Nguyen TT, Breaux C, Kruger C, Stadler K. Diet induced obesity and kidney disease – in search of a susceptible mouse model. Biochimie. 2016;124:65–73. doi: 10.1016/j.biochi.2016.01.012. DOI: https://doi.org/10.1016/j.biochi.2015.08.001
Dicken W, Mehta A, Karagiannis A, Jain V, Vavuranakis M, Sperling L, et al. Statin associated muscle symptoms: An update and review. Prog Cardiovasc Dis. 2022;75:40–48. doi: 10.1016/j.pcad.2022.05.007. DOI: https://doi.org/10.1016/j.pcad.2022.11.010
Maheshwari RA, Sailor GU, Patel L, Balaraman R. Amelioration of cisplatin-induced nephrotoxicity by statins. Indian J Pharmacol. 2013;45(4):354–358. doi: 10.4103/0253-7613.120620. DOI: https://doi.org/10.4103/0253-7613.115016
Iacovelli JJ, Alpenglow JK, Ratchford SM, Craig JC, Simmons JM, Zhao J, et al. Statin administration improves vascular function in heart failure with preserved ejection fraction. J Appl Physiol (1985). 2024;136(4):877–888. doi: 10.1152/japplphysiol.00614.2023. DOI: https://doi.org/10.1152/japplphysiol.00775.2023
Laufs U, Isermann B. Statin intolerance: myths and facts. Eur Heart J. 2020;41(35):3343–3345. doi:10.1093/eurheartj/ehaa693. DOI: https://doi.org/10.1093/eurheartj/ehaa582
Ramachandran R, Manan A, Kim J, Choi S. NLRP3 inflammasome: a key player in the pathogenesis of life style disorders. Exp Mol Med. 2024;56(7):1488–1500. doi: 10.1038/s12276 024 00730 5. DOI: https://doi.org/10.1038/s12276-024-01261-8
Hong CG, Florida E, Li H, Parel PM, Mehta NN, Sorokin AV. Oxidized low density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: a systematic review and meta analysis. Front Cardiovasc Med. 2023;9:1023651. doi:10.3389/fcvm.2022.1023651 DOI: https://doi.org/10.3389/fcvm.2022.1023651
Xie X, Yi W, Zhang P, Wu N, Yan Q, Yang H, et al. Green tea polyphenols, mimicking the effects of dietary restriction, ameliorate high fat diet induced kidney injury via regulating autophagy flux. Nutrients. 2017;9(5):497. doi: 10.3390/nu9050497. DOI: https://doi.org/10.3390/nu9050497
Hu Y, Wang X, Ye L, Li C, Chen W, Cheng H. Rosuvastatin alleviates intestinal injury by down regulating the CD40 pathway in the intestines of rats following traumatic brain injury. Front Neurol. 2020;11:816. doi: 10.3389/fneur.2020.00816. DOI: https://doi.org/10.3389/fneur.2020.00816
Winzer EB, Gaida P, Höllriegel R, Fischer T, Linke A, Schuler G, et al. Impact of Rosuvastatin Treatment on HDL Induced PKC βII and eNOS Phosphorylation in Endothelial Cells and Its Relation to Flow Mediated Dilatation in Patients with Chronic Heart Failure. Cardiol Res Pract. 2016;2016:4826102. doi: 10.1155/2016/4826102. DOI: https://doi.org/10.1155/2016/4826102
Lim AI, Tang SC, Lai KN, Leung JC. Kidney injury molecule 1: More than just an injury marker of tubular epithelial cells? J Cell Physiol. 2013;228(5):917–924. doi: 10.1002/jcp.24254. DOI: https://doi.org/10.1002/jcp.24267
Hammad FT, Lubbad L. The effect of aliskiren on the renal dysfunction following unilateral ureteral obstruction in the rat. Int J Physiol Pathophysiol Pharmacol. 2016;8(2):70-77. PMID: 27570581.
Zhou Y, Lin S, Zhang L, Li Y. Resveratrol prevents renal lipotoxicity in high fat diet treated mouse model through regulating PPAR α pathway. Mol Cell Biochem. 2016;411:143–150. doi:10.1007/s11010 015 2534 7. DOI: https://doi.org/10.1007/s11010-015-2576-y
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











