Pharmacokinetic Study of Oral Disulfiram Suspension and Topical Transdermal Nano-Invasomes Gel in Wistar Rats
DOI:
https://doi.org/10.54133/ajms.v7i1.1130Keywords:
Disulfiram, Invasomes, Transdermal, pharmacokinetics, Wistar ratsAbstract
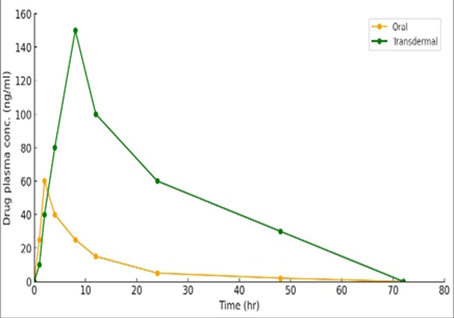
Background: Disulfiram (DSF), an FDA-approved pharmaceutical for the management of alcoholism, has demonstrated its efficacy against several kinds of cancer. DSF has limited solubility, a fast metabolism, a short duration of action, and instability in physiological environments, mostly caused by rapid degradation in the acidic gastric environment. Objective: A transdermal gel containing disulfiram, which was loaded into invasomes, was developed to improve the stability of DSF and enable its effective distribution to tumor tissues. Methods: This study included 72 Wistar rats weighing 200±35 g, which were separated into two groups, each of which included 12 animals. Rats were orally provided a dose of 5 mg of pure DSF suspension via oral gavage, and DSF nano-invasomal transdermal gel was then applied to their skin. DSF is determined in rats' plasma by reverse-phase high-performance liquid chromatography (RP-HPLC). Results: The results showed that the maximum effect (Cmax, Tmax, and AUC0-72) were (Cmax=57.3±0.2, Tmax=3.6±0.01 and 562±3. 3ng.h/ml) for oral and (Cmax=138±0.4, Tmax=5.5±0.01 and 2819±6.6 ng. h/ml) for transdermal routes, respectively. Results showed that the time and concentration needed to achieve the maximum effect (Cmax and Tmax) were significantly different between DSF-oral suspension and transdermal invasomal gel (p<0.05). The relative bioavailability for the transdermal route was five times that of the oral route after a single dose administered for 72 hours. Conclusions: The nano-invasomal transdermal gel filled with DSF demonstrated a more convenient way of administering DSF compared to the oral route.
Downloads
References
Deulkar DA, Kubde JA, Hatwar PR, Bakal RL. A review on transdermal drug delivery system. GSC Advanced Research and Reviews. 2024;18(2):347-361. doi: 10.30574/gscarr.2024.18.2.0052. DOI: https://doi.org/10.30574/gscarr.2024.18.2.0052
Salih O, Muhammed E. Preparation, evaluation, and histopathological studies of ondansetron-loaded invasomes transdermal gel. J Res Pharm. 2024;28(1). doi: 10.29228/jrp.696. DOI: https://doi.org/10.29228/jrp.696
Samir B, El-Kamel A, Zahran N, Heikal L. Resveratrol-loaded invasome gel: A promising nanoformulation for treatment of skin cancer. Drug Del Transl Res. 2024:1-7. doi: 10.1016/j.jddst.2024.105686. DOI: https://doi.org/10.1007/s13346-024-01534-9
Najlah M, Said Suliman A, Tolaymat I, Kurusamy S, Kannappan V, Elhissi AM, et al. Development of injectable PEGylated liposome encapsulating disulfiram for colorectal cancer treatment. Pharmaceutics. 2019;11(11):610. doi: 10.3390/pharmaceutics11110610. DOI: https://doi.org/10.3390/pharmaceutics11110610
Bassani D, Parrott NJ, Manevski N, Zhang JD. Another string to your bow: machine learning prediction of the pharmacokinetic properties of small molecules. Expert Opin Drug Discov. 2024:1-6. doi: 10.1080/17460441.2024.2348157. DOI: https://doi.org/10.1080/17460441.2024.2348157
Suwanpidokkul N, Thongnopnua P, Umprayn K. Transdermal delivery of zidovudine (AZT): the effects of vehicles, enhancers, and polymer membranes on permeation across cadaver pig skin. AAPS PharmSciTech. 2004;5:829. doi: 10.1208/pt050348. DOI: https://doi.org/10.1208/pt050348
Zhang Y, Zhang K, Wang Z, Hu H, Jing Q, Li Y, et al. Transcutol® P/Cremophor® EL/ethyl oleate–formulated microemulsion loaded into hyaluronic acid-based hydrogel for improved transdermal delivery and biosafety of ibuprofen. AAPS PharmSciTech. 2020; 21:1-0. doi: 10.1208/s12249-019-1584-8. DOI: https://doi.org/10.1208/s12249-019-1584-8
National Committee for Research Ethics in Science and Techno. Guidelines For Research Ethics in Science and Technology. JahrbWiss Eth. 2009;14(1). DOI: https://doi.org/10.1515/9783110208856.255
Salih OS, Al-Akkam EJ. Pharmacokinetic parameters of ondansetron in rats after oral solution and transdermal invasomes gel: A comparison study. J Adv Pharm Edu Res. 2023;13(1):117. doi: 10.51847/HS5a27EI6o. DOI: https://doi.org/10.51847/HS5a27EI6o
Almotairy A, Alyahya M, Althobaiti A, Almutairi M, Bandari S, Ashour EA, Repka MA. Disulfiram 3D printed film produced via hot-melt extrusion techniques as a potential anticervical cancer candidate. Int J Pharm. 2023;635:122709. doi: 10.1016/j.ijpharm.2023.122709. DOI: https://doi.org/10.1016/j.ijpharm.2023.122709
Najlah M, Ahmed Z, Iqbal M, Wang Z, Tawari P, Wang W, et al. Development and characterisation of disulfiram-loaded PLGA nanoparticles for the treatment of non-small cell lung cancer. Eur J Pharm Biopharm. 2017;112:224-233. doi: 10.1016/j.ejpb.2016.11.032. DOI: https://doi.org/10.1016/j.ejpb.2016.11.032
Ahmed OAA, Badr-Eldin SM. Development of an optimized avanafil-loaded invasomal transdermal film: Ex vivo skin permeation and in vivo evaluation. Int J Pharm. 2019;570:118657. doi: 10.1016/j.ijpharm.2019.118657. DOI: https://doi.org/10.1016/j.ijpharm.2019.118657
Naji GH, Al-Gawhari FJ. Comparative evaluation of pharmacokinetic parameters between a pure nisoldipine suspension and a nisoldipine-loaded bilosome suspension. Rev. Clin. Pharmacol. Pharmacokinet. Int. Ed. 2024;38(Sup2):149-152. doi: 10.61873/TVTQ4413. DOI: https://doi.org/10.61873/TVTQ4413
Abbas IK, Abd Alhammid SN, Fadhil AA, Hareeja MM. Bioavailability of bilastine oral self-nanoemulsion: Comparative study with commercial formula in rats. Al-Rafidain J Med Sci. 2024;7(1):13-17. doi: 10.54133/ajms.v7i1.1024. DOI: https://doi.org/10.54133/ajms.v7i1.1024
Hashim AA, Rajab NA. Anastrozole loaded nanostructured lipid carriers: Preparation and evaluation. Iraqi J Pharm Sci. 2021;30(2):185-195. doi: 10.31351/vol30iss2pp185-195. DOI: https://doi.org/10.31351/vol30iss2pp185-195
Abdulqader AA, Rajab NA. Bioavailability study of posaconazole in rats after oral poloxamer P188 nano-micelles and oral posaconazole pure drug. J Adv Pharm Edu Res. 2023;13(2):141. doi: 10.51847/Q59uyvRmY3. DOI: https://doi.org/10.51847/Q59uyvRmY3
Al-Akkam EJ, Rasool AA, Badwan AA, Qinna N. Development and validation of a sensitive and accurate method for determination of atorvastatin and rosuvastatin in rat plasma by reversed-phase high-performance liquid chromatography with UV detection. Int J Pharm Pharm Sci. 2013;5:211-219.
Saracino MA, Marcheselli C, Somaini L, Gerra G, De Stefano F, Pieri MC, et al. Simultaneous determination of disulfiram and bupropion in human plasma of alcohol and nicotine abusers. Anal Bioanal Chem. 2010;398:2155-2156. doi: 10.1007/s00216-010-4172-z. DOI: https://doi.org/10.1007/s00216-010-4172-z
Tawfik MA, Tadros MI, Mohamed MI, El-Helaly SN. Low-frequency versus high-frequency ultrasound-mediated transdermal delivery of agomelatine-loaded invasomes: Development, optimization, and in-vivo pharmacokinetic assessment. Int J Nanomed. 2020;15:8893–8910. doi: 10.2147/IJN.S283911. DOI: https://doi.org/10.2147/IJN.S283911
Tawfik MA, Eltaweel MM, Fatouh AM, Shamsel-Din HA, Ibrahim AB. Brain targeting of zolmitriptan via transdermal threesomes: statistical optimization and in vivo biodistribution study by 99mTc radiolabelling technique. Drug Del Transl Res. 2023:1-8. doi: 10.1007/s13346-023-01373-0. DOI: https://doi.org/10.1007/s13346-023-01373-0
Sulaiman HT, Rajab NA. Soluplus and solutol HS-15 olmesartan medoxomil nanomicelle based oral fast dissolving film: in vitro and in vivo characterization. Farmacia. 2024;72(4). doi: 10.31925/farmacia.2024.4.7. DOI: https://doi.org/10.31925/farmacia.2024.4.7
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











