In vivo Brain Pharmacokinetics of Dolutegravir Sodium-Loaded Nanostructured Lipid Carrier in situ Gel: Comparative Study with an Intravenous Drug Solution
DOI:
https://doi.org/10.54133/ajms.v8i1.1692Keywords:
Dolutegravir sodium, HIV, in situ gel, NLCAbstract
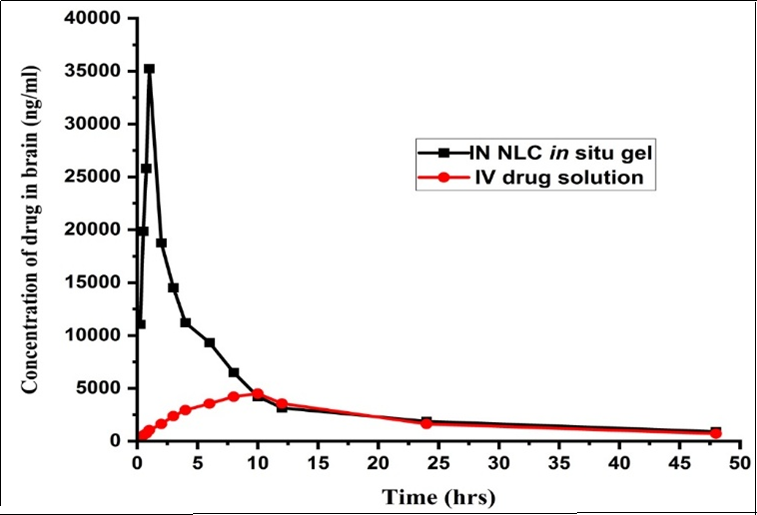
Background: Dolutegravir sodium (DTG), used to treat HIV, faces challenges in delivering effective therapeutic concentrations to the brain due to the blood-brain barrier (BBB). Nanostructured lipid carriers (NLCs) combined with in situ gels present a promising strategy for enhancing brain drug delivery via the intranasal route. Objective: To compare brain pharmacokinetics of DTGs delivered via NLC-loaded in situ gel intranasal administration with the conventional intravenous (IV) drug solution. Methods: 80 Wistar rats, which were divided into three groups: two groups consisting of 39 animals each and a control group with 2 animals. Rats were administered with a dose of 1.0 mg/kg of DTGs IV, and DTGs NLC-loaded in situ gel were administered intranasally. DTGs were determined in rats’ plasma and brain tissue by high-performance liquid chromatography (HPLC). Results: Intranasal administration produced significantly higher brain drug concentrations (Cmax 35344.8ng/ml) compared to the IV solution (Cmax 4536.85ng/ml). The area under the curve (AUC) for the intranasal formulation was twice that of the IV solution, indicating enhanced bioavailability. Furthermore, the intranasal route exhibited a faster onset (lower Tmax) and prolonged retention in brain tissue. The developed nanoformulation exhibited a Drug Targeting Efficiency (DTE) of 232.5% and a Drug Targeting Potential (DTP) of 57%, suggesting improved brain targeting efficiency. Conclusions: The DTGs-loaded NLC in situ gel shows superior brain pharmacokinetics compared to IV administration, highlighting its potential as an effective strategy for enhancing brain targeting.
Downloads
References
Kakad SP, Kshirsagar SJ. Neuro-AIDS: Current status and challenges to antiretroviral drug therapy (ART) for its treatment. CDTH. 2020;15(5):469–481. doi: 10.2174/1574885515666200604123046. DOI: https://doi.org/10.2174/1574885515666200604123046
Taher SS, Al-Kinani KK. Current nanotechnological strategies for delivery of antiretroviral drugs: Overview and future prospects. CDTH. 2024;20. doi: 10.2174/0115748855331460241017100207. DOI: https://doi.org/10.2174/0115748855331460241017100207
Nastri BM, Pagliano P, Zannella C, Folliero V, Masullo A, Rinaldi L, et al. HIV and drug-resistant subtypes. Microorganisms. 2023;11(1):221. doi: 10.3390/microorganisms11010221. DOI: https://doi.org/10.3390/microorganisms11010221
Mastrangelo A, Gama L, Cinque P. Strategies to target the central nervous system HIV reservoir. Curr Opin HIV AIDS. 2024. doi: 10.1097/COH.0000000000000847 DOI: https://doi.org/10.1097/COH.0000000000000847
Jaafer H, Al-Kinani KK. Formulation and evaluation of idebenone microemulsion as a potential approach for the transmucosal drug delivery systems. IJPS. 2024;33(1):79–88. doi: 10.31351/vol33iss1pp79-88. DOI: https://doi.org/10.31351/vol33iss1pp79-88
Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170. doi: 10.1016/j.brainresbull.2018.10.009. DOI: https://doi.org/10.1016/j.brainresbull.2018.10.009
Keller LA, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv and Transl Res. 2022;12(4):735–757. doi: 10.1007/s13346-020-00891-5. DOI: https://doi.org/10.1007/s13346-020-00891-5
Sarma A, Das MK. Nose to brain delivery of antiretroviral drugs in the treatment of neuroAIDS. Mol Biomed. 2020;1(1):15. doi: 10.1186/s43556-020-00019-8. DOI: https://doi.org/10.1186/s43556-020-00019-8
Patharapankal EJ, Ajiboye AL, Mattern C, Trivedi V. Nose-to-brain (N2B) delivery: An alternative route for the delivery of biologics in the management and treatment of central nervous system disorders. Pharmaceutics. 2023;16(1):66. doi: 10.3390/pharmaceutics16010066. DOI: https://doi.org/10.3390/pharmaceutics16010066
Muhammed SA, Al-Kinani KK. Formulation and in vitro evaluation of meloxicam as a self-microemulsifying drug delivery system. F1000Res. 2023;12:315. doi: 10.12688/f1000research.130749.1. DOI: https://doi.org/10.12688/f1000research.130749.2
Jaber SA, Rajab NA. Preparation and In vitro/ex vivo evaluation of nanoemulsion-based in situ gel for intranasal delivery of lasmiditan. Iraqi J Pharm Sci. 2024;33(3):128–141. doi: 10.31351/vol33iss3pp128-141. DOI: https://doi.org/10.31351/vol33iss3pp128-141
Alkufi HK, Kassab HJ. Soluplus-stabilized nimodipine-entrapped spanlastic formulations prepared with edge activator (Tween20): Comparative physicochemical evaluation. Pharm Nanotechnol. 2024;13. doi: 10.2174/0122117385348551241028102256. DOI: https://doi.org/10.2174/0122117385348551241028102256
Shah B, Khunt D, Bhatt H, Misra M, Padh H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: Risk assessment and QbD based optimization. J Drug Deliv Sci Technol. 2016;33:37–50. doi: 10.1016/j.jddst.2016.03.008. DOI: https://doi.org/10.1016/j.jddst.2016.03.008
Tamer MA, Kassab HJ. Optimizing intranasal amisulpride loaded nanostructured lipid carriers: Formulation, development, and characterization parameters. Pharm Nanotechnol. 2024;13(2):287–302. doi: 10.2174/0122117385301604240226111533. DOI: https://doi.org/10.2174/0122117385301604240226111533
Koo J, Lim C, Oh KT. Recent advances in intranasal administration for brain-targeting delivery: A comprehensive review of lipid-based nanoparticles and stimuli-responsive gel formulations. Int J Nanomedicine. 2024;19:1767-1807. doi: 10.2147/IJN.S439181. DOI: https://doi.org/10.2147/IJN.S439181
Alaayedi MH, Maraie NK. Effect of pluronic F127 concentration on gelling temperature and other parameters of lomustine mucoadhesive in-situ gel. Iraqi J Pharm Sci. 2024;33(3):63–71. doi: 10.31351/vol33iss3pp63-71. DOI: https://doi.org/10.31351/vol33iss3pp63-71
Rajab NA, Jawad MS. Preparation and evaluation of rizatriptan benzoate loaded nanostructured lipid carrier using different surfactant/co-surfactant systems. Int J Drug Deliv Technol. 2023;13(01):120–126. doi: 10.25258/ijddt.13.1.18. DOI: https://doi.org/10.25258/ijddt.13.1.18
Belgamwar AV, Khan SA, Yeole PG. Intranasal dolutegravir sodium loaded nanoparticles of hydroxypropyl-beta-cyclodextrin for brain delivery in neuro-AIDS. J Drug Deliv Sci Technol. 2019;52:1008–1020. doi: 10.1016/j.jddst.2019.06.014. DOI: https://doi.org/10.1016/j.jddst.2019.06.014
Patel SH, Ismaiel OA, Mylott WR, Yuan M, Hauser KF, McRae M. Simultaneous determination of intracellular concentrations of tenofovir, emtricitabine, and dolutegravir in human brain microvascular endothelial cells using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Analytica Chimica Acta. 2019;1056:79–87. doi: 10.1016/j.aca.2019.01.015. DOI: https://doi.org/10.1016/j.aca.2019.01.015
Dharshini KP, Devi DR, Banudevi S, Narayanan VHB. In-vivo pharmacokinetic studies of Dolutegravir loaded spray dried Chitosan nanoparticles as milk admixture for paediatrics infected with HIV. Sci Rep. 2022;12(1):13907. doi: 10.1038/s41598-022-18009-x. DOI: https://doi.org/10.1038/s41598-022-18009-x
Sahoo L, Jena GK, Patro CS, Patro CN, Meher NR. In vitro and in vivo characterization of transdermal patch loaded with nanostructured lipid carrier for bioavailability enhancement of dolutegravir sodium using Taguchi and Box-Behnken design. BioNanoSci. 2023;13(3):1213–1230. doi: 10.1007/s12668-023-01143-9. DOI: https://doi.org/10.1007/s12668-023-01143-9
Sonawane D, Pokharkar V. Nose to brain targeting of the donepezil nanostructured lipid carrier in situ gel: formulation, in vitro , ex vivo , in vivo pharmacokinetic and pharmacodynamic characterization. RSC Pharm. 2024;1(4):820–840. doi: 10.1039/D4PM00174E. DOI: https://doi.org/10.1039/D4PM00174E
Mohanty D, Alsaidan OA, Zafar A, Dodle T, Gupta JK, Yasir M, et al. Development of atomoxetine-loaded NLC in situ gel for nose-to-brain delivery: Optimization, in vitro, and preclinical evaluation. Pharmaceutics. 2023;15(7):1985. doi: 10.3390/pharmaceutics15071985. DOI: https://doi.org/10.3390/pharmaceutics15071985
Yaribeygi H, Hemmati MA, Nasimi F, Maleki M, Jamialahmadi T, Reiner I, et al. Sodium glucose cotransporter-2 inhibitor empagliflozin increases antioxidative capacity and improves renal function in diabetic rats. J Clin Med. 2023;12(11):3815. doi: 10.3390/jcm12113815. DOI: https://doi.org/10.3390/jcm12113815
Underwood W, Anthony R. AVMA guidelines for the euthanasia of animals: 2020 edition. Retrieved in March. 2020 Mar;2013(30):2020-1.
Hamzah M, Kassab H. Formulation and characterization of intranasal drug delivery of frovatriptan-loaded binary ethosomes gel for brain targeting. Nanotechnol Sci Appl. 2024;17:1–19. doi: 10.2147/NSA.S442951. DOI: https://doi.org/10.2147/NSA.S442951
Al-Tamimi D, Al-Kinani K, Taher S, Hussein A. Effect of food on the pharmacokinetics of fluoxetine in healthy male adult volunteers. Iraqi J Pharm Sci. 2023;31(Suppl.):153–161. doi: 10.31351/vol31issSuppl.pp153-161. DOI: https://doi.org/10.31351/vol31issSuppl.pp153-161
Ramyasree A, Umadevi S. An efficient RP-HPLC-PDA method for estimating dolutegravir and lamivudine in combined pharmaceutical formulations using a Box-Behnken design approach. Int J Pharm Qual Assur. 2023;14(03):507–513. doi: 10.25258/ijpqa.14.3.08. DOI: https://doi.org/10.25258/ijpqa.14.3.08
Abdelbary GA, Tadros MI. Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers: In vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int J Pharm. 2013;452(1–2):300–310. doi: 10.1016/j.ijpharm.2013.04.084. DOI: https://doi.org/10.1016/j.ijpharm.2013.04.084
Bhandari R, Kuhad A, Paliwal JK, Kuhad A. Development of a new, sensitive, and robust analytical and bio-analytical RP-HPLC method for in-vitro and in-vivo quantification of naringenin in polymeric nanocarriers. J Anal Sci Technol. 2019;10(1):11. doi: 10.1186/s40543-019-0169-1. DOI: https://doi.org/10.1186/s40543-019-0169-1
Nair AB, Chaudhary S, Shah H, Jacob S, Mewada V, Shinu P, et al. Intranasal delivery of darunavir-loaded mucoadhesive in situ gel: Experimental design, in vitro evaluation, and pharmacokinetic studies. Gels. 2022;8(6):342. doi: 10.3390/gels8060342. DOI: https://doi.org/10.3390/gels8060342
Nair AB, Chaudhary S, Jacob S, Patel D, Shinu P, Shah H, et al. Intranasal administration of dolutegravir-loaded nanoemulsion-based in situ gel for enhanced bioavailability and direct brain targeting. Gels. 2023;9(2):130. doi: 10.3390/gels9020130. DOI: https://doi.org/10.3390/gels9020130
Hamzah ML, Kassab HJ. Frovatriptan succinate intranasal delivery for brain targeting – in vivo study. Iraqi J Vet Med. 2023;47(2):101–109. doi: 10.30539/me2mm152. DOI: https://doi.org/10.30539/me2mm152
Singh SK, Dadhania P, Vuddanda PR, Jain A, Velaga S, Singh S. Intranasal delivery of asenapine loaded nanostructured lipid carriers: formulation, characterization, pharmacokinetic and behavioural assessment. RSC Adv. 2016;6(3):2032–22045. doi: 10.1039/C5RA19793G. DOI: https://doi.org/10.1039/C5RA19793G
Noorulla KM, Yasir M, Muzaffar F, Roshan S, Ghoneim MM, Almurshedi AS, et al. Intranasal delivery of chitosan decorated nanostructured lipid carriers of Buspirone for brain targeting: Formulation development, optimization and In-Vivo preclinical evaluation. J Drug Deliv Sci Technol. 2022;67:102939. doi: 10.1016/j.jddst.2021.102939. DOI: https://doi.org/10.1016/j.jddst.2021.102939
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











