Utilization of SuperCYPsPred Software for Predicting Drug Interactions Mediated by Cytochrome P450 Isoenzymes in Elderly Patients Receiving Polypharmacy
DOI:
https://doi.org/10.54133/ajms.v8i1.1763Keywords:
Elderly, Isoenzymes, Interactions, Prevalence, PolypharmacyAbstract
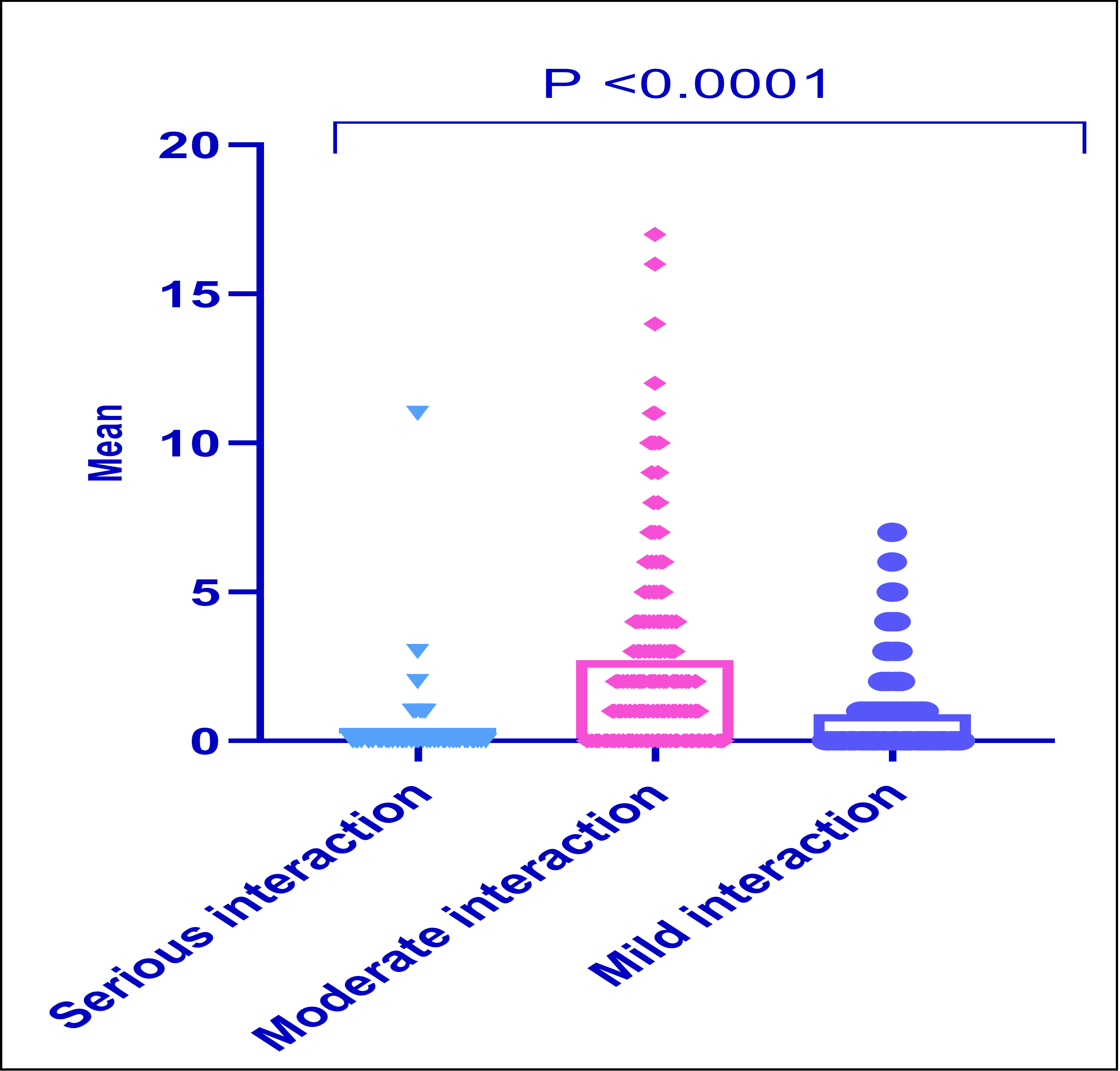
Background: Increasing polypharmacy and complicated prescription regimens raise the likelihood of CYP-mediated drug-drug interactions (DDIs) in older people. Objective: To assess the incidence of CYP-mediated DDIs in older people with polypharmacy and examine the correlation between medication dispensation and the likelihood of these interactions in this high-risk group. Methods: A cross-sectional 17-week analysis was performed, including consecutive new patients aged 65 years and over who were undergoing polypharmacy (defined as the use of more than five medications) at a community pharmacy. The medication profiles of these individuals were evaluated using SuperCYPsPred software and UpToDate® Lexidrug. The frequency of possible CYP-mediated interactions was evaluated. The pharmacists' judgments to suggest prescription adjustments based on the likelihood of CYP-mediated interactions were documented. Results: The prevalence of possible CYP-mediated drug-drug interactions identified among 220 older persons with polypharmacy was 84.5%. Moderate severity DDIs were the predominant and significant kind of interaction (2.70±3.157). A linear regression analysis was performed to predict the frequency of drug interactions based on the number of drugs. A significant association has been identified (F(1,14)= 67.789, p<0.001). The predominant CYP isoenzyme was CYP3A4 at 34.6%, followed by CYP2C9 at 21.4% and CYP2D6 at 15.7%, with no notable gender differences. Conclusion: The older patients have at least one DDI. Elderly adults taking five or more medicines require frequent care owing to a fourfold greater chance of drug interactions.
Downloads
References
Machado J, Rodrigues C, Sousa R, Gomes LM. Drug–drug interaction extraction‐based system: An natural language processing approach. Expert Syst. 2025;42(1):e13303. doi: 10.1111/exsy.13303. DOI: https://doi.org/10.1111/exsy.13303
Stingl JC, Vivian, R. Pharmacogenetic guided drug therapy – how to deal with phenoconversion in polypharmacy. Exp Opi Drug Metab Toxicol. 2025;1–9. doi: 10.1080/17425255.2025.2451440. DOI: https://doi.org/10.1080/17425255.2025.2451440
Jerjes W. Rethinking polypharmacy: empowering junior doctors to tackle a chronic condition in modern practice. Oxford University Press; 2025. p. qgae198. doi:10.1093/postmj/qgae198. DOI: https://doi.org/10.1093/postmj/qgae198
Naharci MI. Frail older adults with high anticholinergic burden are at risk of orthostatic hypotension. J Am Med Assoc. 2025;26(3):105436. doi:10.1016/j.jamda.2024.105436. DOI: https://doi.org/10.1016/j.jamda.2024.105436
Abbas HK, Kadhim DJ, Gorial FI, Shareef LG. Assessment of medication-related burden among a sample of Iraqi patients with systemic lupus erythematosus and its relationship with disease activity: a cross-sectional study. F1000Research. 2022;11(970):970. doi:10.12688/f1000research.124698.1. DOI: https://doi.org/10.12688/f1000research.124698.2
Ugwendum D, Rahman EU, Farah F, Forsah S, Mahmoud M, Agbor DBA. Polypharmacy in patients with chronic kidney disease with cardiovascular disease comorbidities. A systemic review. Int Clin Med Case Rep J. 2024;3(2):1.
Howard AF, Li H, Haljan G. Health equity in the care of adult critical illness survivors. Crit Care Clin. 2025;41(1):185-198. doi: 10.1016/j.ccc.2024.08.010. DOI: https://doi.org/10.1016/j.ccc.2024.08.010
Mair AR, Jordan M, Mullan J. Polypharmacy and deprescribing. Principles Pract Pharcovigil Drug Safe. 2024;405-435. doi:10.1007/978-3-031-51089-2_18. DOI: https://doi.org/10.1007/978-3-031-51089-2_18
Barrio-Cortes J, Castaño-Reguillo A, Benito-Sánchez B, Beca-Martínez MT, Ruiz-Zaldibar C. Utilization of primary healthcare services in patients with multimorbidity according to their risk level by adjusted morbidity groups: A cross-sectional study in Chamartín district (Madrid). Healthcare. 2024;12:270. doi:10.3390/healthcare12020270. DOI: https://doi.org/10.3390/healthcare12020270
Schroeter T, Lapham K, Varma MVS, Obach RS. Positioning enzyme-and transporter-based precipitant drug–drug interaction studies in drug design. J Med Chem. 2025;68(2):1021–1032. doi:10.1021/acs.jmedchem.4c02629. DOI: https://doi.org/10.1021/acs.jmedchem.4c02629
Hasan MK, Ghareeb MM, Mate BF, Aga QAIAK. Clinical adverse effects of chemotherapy protocolusing 6-mercaptopurine in Iraqi patients with acute lymphocytic leukemia during maintenance phase. Res J Pharm Technol. 2019;12(12):5757-64. doi:10.5958/0974-360X.2019.00997.1. DOI: https://doi.org/10.5958/0974-360X.2019.00997.1
Hughes JE, Menditto E, Mucherino S, Orlando V, Moreno-Juste A, Gimeno-Miguel A, et al. The European drug-drug interaction (EuroDDI) study protocol: A cross-country comparison of drug-drug interaction prevalence in the older community-dwelling population. Pharmacoepidemiol Drug Saf. 2025;34(1):e70092. doi: 10.1002/pds.70092. DOI: https://doi.org/10.1002/pds.70092
Pedersen KW, Andersen JD, Hansen J, Børsting C, Banner J, Hasselstrøm JB, et al. Investigating the correlation between genotypes and hepatic protein expression of CYP2C9, CYP2C19, CYP2D6, and CYP3A5 using postmortem tissue from a Danish population. Drug Metab Dispos. 2024;52(9):975-980. doi: 10.1124/dmd.124.001692. DOI: https://doi.org/10.1124/dmd.124.001692
Kmieć JW, Kulej P, Woźniak J, Biesiada W, Chernysh A-M, Dusińska A, et al. The role of physical activity in reducing inflammation: Implications for preventive medicine. Qual Sport. 2025;37:57102. doi: 10.12775/qs.2025.37.57102. DOI: https://doi.org/10.12775/QS.2025.37.57102
Wannawichate T, Limpawattana P. A comparative analysis of the drug interaction programmes amongst geriatric outpatients. Indian J Physiol Pharmacol. 2025:1-6. doi: 10.25259/IJPP_590_2023. DOI: https://doi.org/10.25259/IJPP_590_2023
Lin CF, Wang CY, Bai CH. Polypharmacy, aging and potential drug-drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28:219-225. doi: 10.2165/11586870-000000000-00000. DOI: https://doi.org/10.2165/11586870-000000000-00000
Salwe KJ, Kalyansundaram D, Bahurupi Y. A study on polypharmacy and potential drug-drug interactions among elderly patients admitted in department of medicine of a tertiary care hospital in Puducherry. J Clin Diagnost Res. 2016;10(2):FC06. doi: 10.7860/jcdr/2016/16284.7273. DOI: https://doi.org/10.7860/JCDR/2016/16284.7273
Kashyap M, D’Cruz S, Sachdev A, Tiwari P. Drug-drug interactions and their predictors: Results from Indian elderly inpatients. J Pharm Pract. 2013;11(4):191. DOI: https://doi.org/10.4321/S1886-36552013000400003
Bogetti-Salazar M, Gonzalez-Gonzalez C, Juarez-Cedillo T, Sánchez-García S, Rosas-Carrasco O. Severe potential drug-drug interactions in older adults with dementia and associated factors. Clinics. 2016;71(1):17-21. doi: 10.6061/clinics/2016(01)04. DOI: https://doi.org/10.6061/clinics/2016(01)04
Mendes-Netto RS, Silva CQV, Oliveira Filho AD, Rocha CE, Lyra-Junior DP. Assessment of drug interactions in elderly patients of a family health care unit in Aracaju (Brazil): a pilot study. Afr J Pharm Pharmacol. 2011;5(7):812-818. doi: 10.5897/ajpp10.299.
Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin. Drug Safe. 2014;13(1):57-65. doi: 10.1517/14740338.2013.827660. DOI: https://doi.org/10.1517/14740338.2013.827660
Raschi E, Piccinni C, Signoretta V, Lionello L, Bonezzi S, Delfino M, et al. Clinically important drug–drug interactions in poly‐treated elderly outpatients: a campaign to improve appropriateness in general practice. Br J Clin Pharmacol. 2015;80(6):1411-1420. doi: 10.1111/bcp.12754. DOI: https://doi.org/10.1111/bcp.12754
Linfield RY, Nguyen NN, Laprade OH, Holodniy M, Chary A. An update on drug-drug interactions in older adults living with human immunodeficiency virus (HIV). Expert Rev Clin Pharmacol. 2024;17(7):589–614. doi: 10.1080/17512433.2024.2350968. DOI: https://doi.org/10.1080/17512433.2024.2350968
Munsour EE, Mahmoud MA, Hussain R. Understanding the concepts of health literacy in the context of medication safety. Health Literacy in Medicines Use and Pharmacy: Elsevier; 2025. p. 307-20. doi: 10.1016/B978-0-12-824407-4.00002-7. DOI: https://doi.org/10.1016/B978-0-12-824407-4.00002-7
Shareef LG, Al-Hussainy AF, Hameed SM. COVID-19 vaccination hesitancy among Iraqi general population between beliefs and barriers: An observational study. F1000Research. 2022;11. doi: 10.12688/f1000research.110545.2. DOI: https://doi.org/10.12688/f1000research.110545.1
Nobili A, Pasina L, Tettamanti M, Lucca U, Riva E, Marzona I, et al. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther. 2009;34(4):377-386. doi: 10.1111/j.1365-2710.2009.01021.x. DOI: https://doi.org/10.1111/j.1365-2710.2009.01021.x
Konstandi M, Johnson EO. Age-related modifications in CYP-dependent drug metabolism: role of stress. Front Endocrinol. 2023;14:1143835. doi: 10.3389/fendo.2023.1143835. DOI: https://doi.org/10.3389/fendo.2023.1143835
Nagappa AN, Kanoujia J. Clinical Pharmacy Services: Drug and Poison Information, Ward Round Participation, Drug-Drug Interaction and Drug-Food Interaction, Prescription Analysis, PTC Activities, Formulary Management, and TDM Services. Perspectives in Pharmacy Practice: Trends in Pharmaceutical Care, Springer; 2022. p. 87-109. doi: 10.1007/978-981-16-9213-0_7. DOI: https://doi.org/10.1007/978-981-16-9213-0_7
Zarrabi S, Hosseini E, Sadeghi K, Vaezi M, Shahrami B. Assessment of drug-drug interactions among patients with hematologic malignancy: A clinical pharmacist-led study. J Oncol Pharm Pract. 2024:10781552241281664. doi: 10.1177/10781552241281664. DOI: https://doi.org/10.1177/10781552241281664
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











