Binary Ethosomal Gel for Enhanced Transdermal Delivery of Tazarotene: Development, Refinement, in vitro Evaluation, and Skin Penetration Investigations
DOI:
https://doi.org/10.54133/ajms.v5i1S.288Keywords:
Binary ethosomes, Full factorial design, Melanoma, Tazarotene, Transdermal drug deliveryAbstract
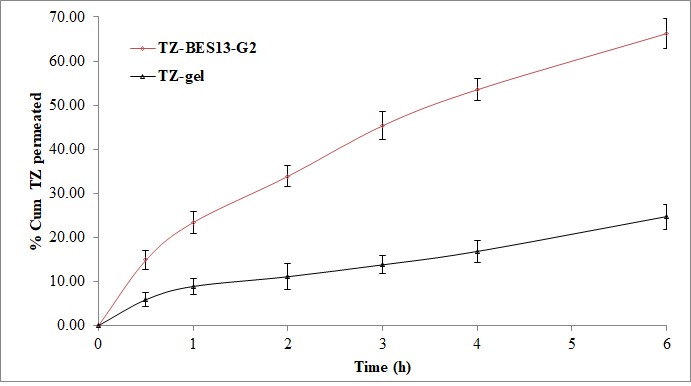
Background: Tazarotene (TZ) is a novel acetylenic class retinoid that selectively targets RARβ/γ. It is not particularly soluble or bioavailable, yet it is used to treat melanoma. Objective: To improve the tazarotene gel formula's transdermal distribution. Methods: TZ-incorporated binary ethosomes (TZ-BES) were developed for the current study. The cold technique and optimized Box-Behnken statistical design tools were used to synthesize the TZ-BES. The improved ethosome (TZ-BES13) was mixed with carbopol gel and tested for stability and ex vivo skin penetration, as well as viscosity, pH, spreadability, and drug content. Results: The optimal ethosomes (TZ-BES13) had a vesicle size of 168 nm, a PDI of 0.367, a zeta potential of -30 mV, and an entrapment effectiveness of 79.94%. TZ is enclosed in the ethosome matrix, as seen by the differential scanning calorimetry thermogram. FTIR shows that the TZ and additives are compatible. TZ-BES13-G2, the optimized TZ-BES13 gel, has a spreadability of 7.82 cm2, a pH of 6.52, a viscosity of 17235, and a drug content of 99.82±1.04%. Compared to the plan TZ-gel (43.54%), the TZ-BES13-G2 exhibits a much higher TZ release (89.22%). In 6 hours, rat abdomen skin permeability for TZ-BES13-G2 was 66.22±3.31%, much greater than that of plan TZ-gel (24.67%). The flow of TZ-BES13-G2 was 2.68 times greater than that of plan TZ-gel. The stability analysis showed that the formulation's properties had not changed significantly. Conclusion: Ethosomal gel offers an alternative mode of TZ administration when used topically.
Downloads
References
Ahmed B, Qadir MI, Ghafoor S. Malignant melanoma: Skin cancer-diagnosis, prevention, and treatment. Crit Rev Eukaryot Gene Expr. 2020;30(4):291-297. doi: 10.1615/CritRevEukaryotGeneExpr.2020028454. DOI: https://doi.org/10.1615/CritRevEukaryotGeneExpr.2020028454
Ścieżyńska A, Sobiepanek A, Kowalska PD, Soszyńska M, Łuszczyński K, Grzywa TM, et al. A novel and effective method for human primary skin melanocytes and metastatic melanoma cell isolation. Cancers. 2021;13(24):6244. doi: 10.3390/cancers13246244. DOI: https://doi.org/10.3390/cancers13246244
Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13(9):E59. PMID: 33133344.
Wu CS, Chen GS, Lin PY, Pan IH, Wang ST, Lin SH, et al. Tazarotene induces apoptosis in human basal cell carcinoma via activation of caspase-8/t-Bid and the reactive oxygen species-dependent mitochondrial pathway. DNA Cell Biol. 2014;33(10):652-666. doi: 10.1089/dna.2014.2366. DOI: https://doi.org/10.1089/dna.2014.2366
Aggarwal G. Topical nano drug delivery for treatment of psoriasis: Progressive and novel delivery. Asian J Pharmaceutics. 2018;12(03): 12(3):S835-S848.
Kumar N, Goindi S. Development, characterization and preclinical evaluation of nanosized liposomes of itraconazole for topical application: 32 full factorial design to estimate the relationship between formulation components. J Drug Deliv Sci Technol. 2021;66:102785. doi: 10.1016/j.jddst.2021.102785. DOI: https://doi.org/10.1016/j.jddst.2021.102785
El-Enin ASMA, Khalifa MKA, Dawaba AM, Dawaba HM. Proniosomal gel-mediated topical delivery of fluconazole: Development, in vitro characterization, and microbiological evaluation. J Adv Pharm Technol Res. 2019;10(1):20. doi: 10.4103/japtr.JAPTR_332_18. DOI: https://doi.org/10.4103/japtr.JAPTR_332_18
Garg AK, Maddiboyina B, Alqarni MHS, Alam A, Aldawsari HM, Rawat P, et al. Solubility enhancement, formulation development and antifungal activity of luliconazole niosomal gel-based system. J Biomater Sci Polym Ed. 2021;32(8):1009-23. doi: 10.1080/09205063.2021.1892471. DOI: https://doi.org/10.1080/09205063.2021.1892471
Nemr AA, El-Mahrouk GM, Badie HA. Development and evaluation of proniosomes to enhance the transdermal delivery of cilostazole and to ensure the safety of its application. Drug Dev Ind Pharm. 2021;47(3):403-415. doi: 10.1080/03639045.2021.1890111. DOI: https://doi.org/10.1080/03639045.2021.1890111
Hajare A, Dol H, Patil K. Design and development of terbinafine hydrochloride ethosomal gel for enhancement of transdermal delivery: In vitro, in vivo, molecular docking, and stability study. J Drug Deliv Sci Technol. 2021;61:102280. doi: 10.1016/j.jddst.2020.102280. DOI: https://doi.org/10.1016/j.jddst.2020.102280
Balata GF, Faisal MM, Elghamry HA, Sabry SA. Preparation and characterization of ivabradine HCl transfersomes for enhanced transdermal delivery. J Drug Deliv Sci Technol. 2020;60:101921. doi: 10.1016/j.jddst.2020.101921. DOI: https://doi.org/10.1016/j.jddst.2020.101921
Jafari A, Daneshamouz S, Ghasemiyeh P, Mohammadi-Samani S. Ethosomes as dermal/transdermal drug delivery systems: applications, preparation and characterization. J Liposome Res. 2023;33(1):34-52. doi: 10.1080/08982104.2022.2085742. DOI: https://doi.org/10.1080/08982104.2022.2085742
Nainwal N, Jawla S, Singh R, Saharan VA. Transdermal applications of ethosomes–a detailed review. J Liposome Res. 2019;29(2):103-113. doi: 10.1080/08982104.2018.1517160. DOI: https://doi.org/10.1080/08982104.2018.1517160
Abdulbaqi IM, Darwis Y, Khan NA, Assi RA, Khan AA. Ethosomal nanocarriers: the impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int J Nanomedicine. 2016;11:2279-2304. doi: 10.2147/IJN.S105016. DOI: https://doi.org/10.2147/IJN.S105016
Moolakkadath T, Aqil M, Ahad A, Imam SS, Praveen A, Sultana Y, et al. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int J Pharm. 2019;560:78-91. doi: 10.1016/j.ijpharm.2019.01.067. DOI: https://doi.org/10.1016/j.ijpharm.2019.01.067
Jeong WY, Kwon M, Choi HE, Kim KS. Recent advances in transdermal drug delivery systems: A review. Biomater Res. 2021;25:1-15. doi: 10.1186/s40824-021-00226-6. DOI: https://doi.org/10.1186/s40824-021-00226-6
Shukla R, Tiwari G, Tiwari R, Rai AK. Formulation and evaluation of the topical ethosomal gel of melatonin to prevent UV radiation. J Cosmet Dermatol. 2020;19(8):2093-2104. doi: 10.1111/jocd.13251. DOI: https://doi.org/10.1111/jocd.13251
Ferrara F, Benedusi M, Sguizzato M, Cortesi R, Baldisserotto A, Buzzi R, et al. Ethosomes and transethosomes as cutaneous delivery systems for quercetin: A preliminary study on melanoma cells. Pharmaceutics. 2022;14(5):1038. doi: 10.3390/pharmaceutics14051038. DOI: https://doi.org/10.3390/pharmaceutics14051038
Arora D, Nanda S. Quality by design driven development of resveratrol loaded ethosomal hydrogel for improved dermatological benefits via enhanced skin permeation and retention. Int J Pharm. 2019;567:118448. doi: 10.1016/j.ijpharm.2019.118448. DOI: https://doi.org/10.1016/j.ijpharm.2019.118448
Dahash RA, Rajab NA, Almajidi YQ. Investigation of the effect of variable components on the preparation and in-vitro evaluation of lacidipine as an oral nanoemulsion dosage form. Int J Drug Deliv Technol. 2021;11(3):1031-1036.
Almajidi YQ, Maraie NK, Raauf AM. Utilization of solid in oil nanodispersion to prepare a topical vemurafenib as potential delivery system for skin melanoma. Appl Nanosci. 2023;13(4):2845-2856. doi: 10.1007/s13204-021-02158-y. DOI: https://doi.org/10.1007/s13204-021-02158-y
Almajidi YQ, Maraie NK, Raauf AM. Modified solid in oil nanodispersion containing vemurafenib-lipid complex-in vitro/in vivo study. F1000Res. 2022;11:841. doi: 10.12688/f1000research. DOI: https://doi.org/10.12688/f1000research.123041.2
Muslim RK, Maraie NK. Preparation and evaluation of nano-binary ethosomal dispersion for flufenamic acid. MaterialsToday: Proceedings. 2022;57:354-361. doi: 10.1016/j.matpr.2021.09.239. DOI: https://doi.org/10.1016/j.matpr.2021.09.239
Fathalla D, Youssef EM, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics. 2020;12(5):446. doi: 10.3390/pharmaceutics12050446. DOI: https://doi.org/10.3390/pharmaceutics12050446
Maraie NK, Almajidi YQ. Effect of different mucoadhesive polymers on release of ondansetron HCl from intranasal mucoadhesive in situ gel. Al Mustansiriyah J Pharm Sci. 2017;17(2):10. doi: 10.32947/ajps.v17i2.47.
Maraie NK, Almajidi YQ. Application of nanoemulsion technology for preparation and evaluation of intranasal mucoadhesive nano-in-situ gel for ondansetron HCl. J Glob Pharma Technol. 2018;10(03):431-442. DOI: https://doi.org/10.32947/ajps.v17i2.47
Rapalli VK, Singhvi G, Srividya G, Waghule T, Dubey S, Saha R, et al. Stability indicating liquid chromatographic method for simultaneous quantification of betamethasone valerate and tazarotene in in‐vitro and ex‐vivo studies of complex nanoformulation. J Sep Sci. 2019;42:3413-3420. doi: 10.1002/jssc.201900538. DOI: https://doi.org/10.1002/jssc.201900538
Chen J-G, Liu Y-F, Gao T-W. Preparation and anti-inflammatory activity of triptolide ethosomes in an erythema model. J Liposome Res. 2010;20(4):297-303. doi: 10.3109/08982100903544144. DOI: https://doi.org/10.3109/08982100903544144
Maritim S, Boulas P, Lin Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int J Pharm. 2021;592:120051. doi: 10.1016/j.ijpharm.2020.120051. DOI: https://doi.org/10.1016/j.ijpharm.2020.120051
Rushmi ZT, Akter N, Mow R, Afroz M, Kazi M, Matas M, et al. The impact of formulation attributes and process parameters on black seed oil loaded liposomes and their performance in animal models of analgesia. Saudi Pharm J. 2016;25. doi: 10.1016/j.jsps.2016.09.011. DOI: https://doi.org/10.1016/j.jsps.2016.09.011
Ma H, Guo D, Fan Y, Wang J, Cheng J, Zhang X. Paeonol-loaded ethosomes as transdermal delivery carriers: design, preparation and evaluation. Molecules. 2018;23(7):1756. doi: 10.3390/molecules23071756. DOI: https://doi.org/10.3390/molecules23071756
Alshehri S, Hussain A, Altamimi MA, Ramzan M. In vitro, ex vivo, and in vivo studies of binary ethosomes for transdermal delivery of acyclovir: A comparative assessment. J Drug Deliv Sci Technol. 2021;62:102390. doi: 10.1016/j.jddst.2021.102390. DOI: https://doi.org/10.1016/j.jddst.2021.102390
Peppas NA. 1. Commentary on an exponential model for the analysis of drug delivery: Original research article: a simple equation for description of solute release: I II. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs, 1987. J Control Release. 2014;190:31-32. PMID: 25356469.
Mbah CC, Builders PF, Attama AA. Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Expert Opin Drug Deliv. 2014;11(1):45-59. doi: 10.1517/17425247.2013.860130. DOI: https://doi.org/10.1517/17425247.2013.860130
David SRN, Hui MS, Pin CF, Ci FY, Rajabalaya R. Formulation and in vitro evaluation of ethosomes as vesicular carrier for enhanced topical delivery of isotretinoin. Int J Drug Deliv. 2013;5(1):28.
El-Shenawy AA, Abdelhafez WA, Ismail A, Kassem AA. Formulation and characterization of nanosized ethosomal formulations of antigout model drug (febuxostat) prepared by cold method: In vitro/ex vivo and in vivo assessment. AAPS PharmSciTech. 2019;21(1):31. doi: 10.1208/s12249-019-1556-z. DOI: https://doi.org/10.1208/s12249-019-1556-z
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











