Formulation and Characterization of Novel Itraconazole-Loaded PLGA Nanoparticles for Potential Topical Delivery
DOI:
https://doi.org/10.54133/ajms.v9i2.2513Keywords:
Itraconazole, Formulation Optimization, Polymeric Nanoparticles, PLGA 75:25Abstract
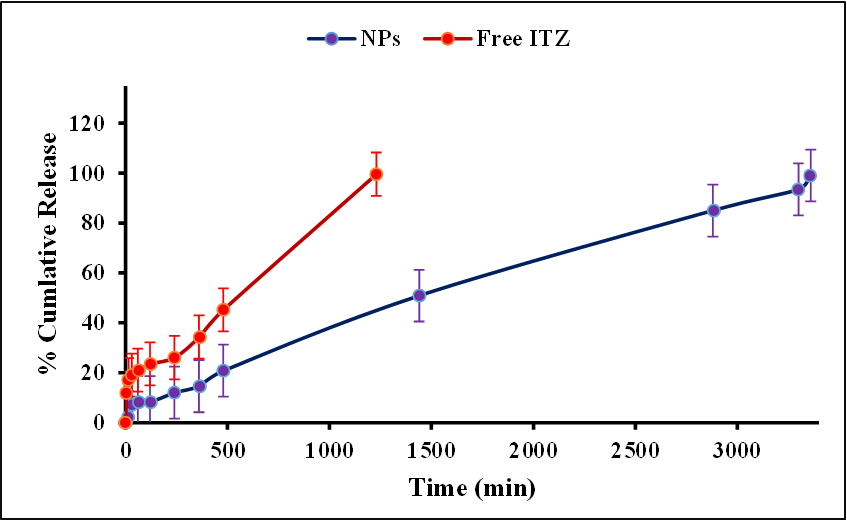
Background: Itraconazole (ITZ) is a potent antifungal drug limited by poor water solubility and systemic side effects when taken orally. Topical administration offers a safer approach; however, efficient penetration through the stratum corneum remains a challenge. Objective: To develop and optimize ITZ-loaded PLGA nanoparticles (NPs) by investigating the effects of polymer type (PLGA 50:50 and 75:25) and stabilizers (Tween-80 and PVA) on the NPs size, PDI, and zeta potential, and to evaluate in vitro drug release of free ITZ and NPs. Methods: NPs were prepared by the nanoprecipitation method and were characterized using DLS for particle size, PDI, and zeta potential; FTIR spectroscopy for confirmation of drug encapsulation; FESEM and TEM for morphological assessment; and in vitro drug release studies. Results: All NP formulations were in the nano-size range. The optimal formulation, containing PLGA 75:25 and 1% Tween-80, had the smallest particle size and lowest PDI, with an encapsulation efficiency of 54.74%, a drug loading capacity of 8.46%, and a yield of 58.77%. FTIR spectroscopy confirmed successful incorporation of ITZ into the PLGA matrix, whereas FESEM and TEM revealed smooth, spherical NPs with uniform distribution and no aggregation. In vitro drug release studies of the NPs demonstrated a biphasic profile: an initial burst followed by a controlled release, as opposed to the rapid release of free ITZ. Conclusions: The NPs were successfully prepared. The optimized ITZ-loaded PLGA nanoparticles showed favorable physicochemical characteristics, efficient encapsulation, and controlled drug release, highlighting potential as a topical delivery system for antifungal therapy.
Downloads
References
Bootman JL. Cost-effectiveness of two new treatments for onychomycosis: An analysis of two comparative clinical trials. J Am Acad Dermatol. 1998;38(5, Supplement 2):S69-S72. doi: 10.1016/S0190-9622(98)70488-8. DOI: https://doi.org/10.1016/S0190-9622(98)70488-8
Islam TAB, Majid F, Ahmed M, Afrin S, Jhumky T, Ferdouse F. Prevalence of dermatophytic infection and detection of dermatophytes by microscopic and culture methods. J Enam Med Coll. 2018;8(1):11-15. doi: 10.3329/jemc.v8i1.35429. DOI: https://doi.org/10.3329/jemc.v8i1.35429
Kumar N, Goindi S. Statistically designed nonionic surfactant vesicles for dermal delivery of itraconazole: Characterization and in vivo evaluation using a standardized Tinea pedis infection model. Int J Pharm. 2014;472(1):224-240. doi: 10.1016/j.ijpharm.2014.06.030. DOI: https://doi.org/10.1016/j.ijpharm.2014.06.030
Dhakane SS, Pandit AP. Novel itraconazole nano-spanlastics gel with enhanced penetration to treat topical fungal infection. J Drug Deliv Sci Technol. 2023;90:105093. doi: 10.1016/j.jddst.2023.105093. DOI: https://doi.org/10.1016/j.jddst.2023.105093
Chen Y, Feng X, Meng S. Site-specific drug delivery in the skin for the localized treatment of skin diseases. Expert Opin Drug Deliv. 2019;16(8):847-867. doi: 10.1080/17425247.2019.1645119. DOI: https://doi.org/10.1080/17425247.2019.1645119
El-Housiny S, Shams Eldeen MA, El-Attar YA, Salem HA, Attia D, Bendas ER, et al. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: formulation and clinical study. Drug Deliv. 2018;25(1):78-90. doi: 10.1080/10717544.2017.1413444 DOI: https://doi.org/10.1080/10717544.2017.1413444
Mathur M, Devi VK. Potential of novel drug delivery systems in the management of topical candidiasis. J Drug Target. 2017;25(8):685-703. doi: 10.1080/1061186x.2017.1331352 DOI: https://doi.org/10.1080/1061186X.2017.1331352
Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77-92. doi: 10.1016/j.jconrel.2015.10.04. DOI: https://doi.org/10.1016/j.jconrel.2015.10.049
Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001;46(1-3):149-168. doi: 10.1016/s0169-409x(00)00131-9. DOI: https://doi.org/10.1016/S0169-409X(00)00131-9
Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185-198. doi: 10.1146/annurev-med-040210-162544. DOI: https://doi.org/10.1146/annurev-med-040210-162544
Abbott LC, Maynard AD. Exposure assessment approaches for engineered nanomaterials. Risk Anal. 2010;30(11):1634-644. doi: 10.1111/j.1539-6924.2010.01446.x. DOI: https://doi.org/10.1111/j.1539-6924.2010.01446.x
Abdelhalim MA. Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels. Lipids Health Dis. 2011;10:233. doi: 10.1186/1476-511x-10-233. DOI: https://doi.org/10.1186/1476-511X-10-233
Dey S, Mazumder B, Pathak Y, (Eds.), Models for risk assessments of nanoparticles, 2014. p. 383-424. doi: 10.1201/b17191-20. DOI: https://doi.org/10.1201/b17191-20
Grognet JM. Nanotechnologies: from information sciences to pharmacology. Therapie. 2008;63(1):1-9. doi: 10.2515/therapie:2008003. DOI: https://doi.org/10.2515/therapie:2008003
Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13(12):1838-1845. doi: 10.1023/a:1016085108889. DOI: https://doi.org/10.1023/A:1016085108889
Alhowyan AA, Altamimi MA, Kalam MA, Khan AA, Badran M, Binkhathlan Z, et al. Antifungal efficacy of Itraconazole loaded PLGA-nanoparticles stabilized by vitamin-E TPGS: In vitro and ex vivo studies. J Microbiol Methods. 2019;161:87-95. doi: 10.1016/j.mimet.2019.01.020. DOI: https://doi.org/10.1016/j.mimet.2019.01.020
Patel NR, Damann K, Leonardi C, Sabliov CM. Itraconazole-loaded poly(lactic-co-glycolic) acid nanoparticles for improved antifungal activity. Nanomedicine (Lond). 2010;5(7):1037-1050. doi: 10.2217/nnm.10.68. DOI: https://doi.org/10.2217/nnm.10.68
Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J Controll Rel. 1999;57(2):171-85. doi: 10.1016/S0168-3659(98)00116-3. DOI: https://doi.org/10.1016/S0168-3659(98)00116-3
Essa S, Louhichi F, Raymond M, Hildgen P. Improved antifungal activity of itraconazole-loaded PEG/PLA nanoparticles. J Microencapsul. 2013;30(3):205-217. doi: 10.3109/02652048.2012.714410. DOI: https://doi.org/10.3109/02652048.2012.714410
Zhang Y, Huo M, Zhou J, Zou A, Li W, Yao C, et al. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010;12(3):263-271. doi: 10.1208/s12248-010-9185-1. DOI: https://doi.org/10.1208/s12248-010-9185-1
Zuo J, Gao Y, Bou-Chacra N, Löbenberg R. Evaluation of the DDSolver software applications. Biomed Res Int. 2014;2014:204925. doi: 10.1155/2014/204925. DOI: https://doi.org/10.1155/2014/204925
Gamal A, Saeed H, El-Ela FIA, Salem HF. Improving the antitumor activity and Bioavailability of sonidegib for the treatment of skin cancer. Pharmaceutics. 2021;13(10):1560. doi: 10.3390/pharmaceutics13101560. DOI: https://doi.org/10.3390/pharmaceutics13101560
Ekambaram P, Abdul HS. Formulation and evaluation of solid lipid nanoparticles of ramipril. J Young Pharm. 2011;3(3):216-220. doi:10.4103/0975-1483.83765. DOI: https://doi.org/10.4103/0975-1483.83765
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6(6):714-729. doi: 10.1016/j.nano.2010.05.005. DOI: https://doi.org/10.1016/j.nano.2010.05.005
Sawant KK, Dodiya SS. Recent advances and patents on solid lipid nanoparticles. Recent Pat Drug Deliv Formul. 2008;2(2):120-135. doi: 10.2174/187221108784534081. DOI: https://doi.org/10.2174/187221108784534081
Quaglia F, Ostacolo L, Mazzaglia A, Villari V, Zaccaria D, Sciortino MT. The intracellular effects of non-ionic amphiphilic cyclodextrin nanoparticles in the delivery of anticancer drugs. Biomaterials. 2009;30(3):374-382. doi: 10.1016/j.biomaterials.2008.09.035. DOI: https://doi.org/10.1016/j.biomaterials.2008.09.035
de la Calle I, Soto-Gómez D, Pérez-Rodríguez P, López-Periago JE. Particle size characterization of Sepia ink eumelanin biopolymers by SEM, DLS, and AF4-MALLS: A comparative study. Food Anal Methods. 2019;12(5):1140-1151. doi: 10.1007/s12161-019-01448-0. DOI: https://doi.org/10.1007/s12161-019-01448-0
Magenheim B, Levy MY, Benita S. A new in vitro technique for the evaluation of drug release profile from colloidal carriers - ultrafiltration technique at low pressure. Int J Pharm. 1993;94(1):115-123. doi: 10.1016/0378-5173(93)90015-8. DOI: https://doi.org/10.1016/0378-5173(93)90015-8
Keles H, Naylor A, Clegg F, Sammon C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polymer Degrad Stability. 2015;119:228-241. doi: 10.1016/j.polymdegradstab.2015.04.025. DOI: https://doi.org/10.1016/j.polymdegradstab.2015.04.025
Baggi R, Kilaru N. Calculation of predominant drug release mechanism using Peppas-Sahlin model, Part-I (substitution method): A linear regression approach. Asian J Pharm Technol. 2016;6:223. doi: 10.5958/2231-5713.2016.00033.7. DOI: https://doi.org/10.5958/2231-5713.2016.00033.7
Peppas NA, Sahlin JJ. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int J Pharm. 1989;57(2):169-172. doi: 10.1016/0378-5173(89)90306-2. DOI: https://doi.org/10.1016/0378-5173(89)90306-2
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











