Stevens-Johnson Syndrome with a 24-Hour Onset Following a Single Dose of Ibuprofen: A Case Report and Review
DOI:
https://doi.org/10.54133/ajms.v9i2.2516Keywords:
Female, Ibuprofen, Non-steroidal anti-inflammatory drugs, Stevens-Johnson syndrome, Side effectsAbstract
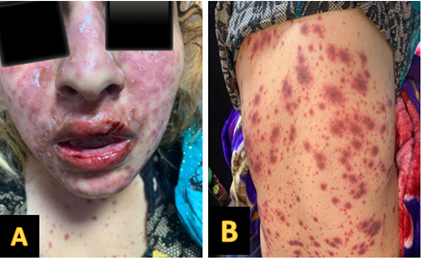
Stevens-Johnson syndrome (SJS) is a rare, severe mucocutaneous disease. Most commonly triggered by infections or prolonged drug use, and reports of such atypical triggers are limited, here we present a case report of a female patient who developed rapid-onset SJS after a single dose of ibuprofen. A 21-year-old woman attended the Emergency Department (ER) with a diffused erythematous, crusted skin lesion associated with painful oral and genital mucosal erosions 24 hours after a single dose of ibuprofen. She was mildly feverish and anxious; her lab test was unremarkable. In the hospital, she received supportive therapy, local skin care, and systemic steroid 60 mg/kg. She gradually improved, and 10 days after admission, she was discharged home. Even a single dose of ibuprofen can trigger SJS, so clinicians must maintain high vigilance, as early recognition and prompt management are critical to prevent severe complications and improve outcomes.
Downloads
References
Shao Q, Yin X, Zeng N, Zhou Z, Mao X, Zhu Y, et al. Stevens-Johnson syndrome following non-steroidal anti-inflammatory drugs: A real-world analysis of post-marketing surveillance data. Front Pediatr. 2022;10:896867. doi: 10.3389/fped.2022.896867. DOI: https://doi.org/10.3389/fped.2022.896867
Fetriani U, Zakiawati D. Viral triggers exposed: A systematic review of virus-induced Stevens-Johnson syndrome/Toxic epidermal necrolysis. J Inflamm Res. 2025;18:12575-12588. doi: 10.2147/JIR.S546186. DOI: https://doi.org/10.2147/JIR.S546186
Fletcher G, Ryan D, Bunker C. Association between Stevens-Johnson syndrome and toxic epidermal necrolysis with Ibuprofen: A pharmacovigilance study in the UK Yellow Card scheme and systematic review of case reports. medRxiv. 2023;2023.12.05.23299283. doi: 10.1101/2023.12.05.23299283. DOI: https://doi.org/10.1101/2023.12.05.23299283
Justice J, Mukherjee E, Martin-Pozo M, Phillips E. Updates in the pathogenesis of SJS/TEN. Allergol Int. 2025;74(3):361-371. doi: 10.1016/j.alit.2025.05.002. DOI: https://doi.org/10.1016/j.alit.2025.05.002
Hasegawa A, Abe R. Stevens–Johnson syndrome and toxic epidermal necrolysis: Updates in pathophysiology and management. Chin Med J. 2024;137(19):2294-2307. doi: 10.1097/CM9.0000000000003250. DOI: https://doi.org/10.1097/CM9.0000000000003250
Yao LM, Su X, Liu LL, Qi YN, Wei B, Ma R, et al. Recent developments in the research of Stevens-Johnson syndrome and toxic epidermal necrolysis: pathogenesis, diagnosis and treatment. Eur J Med Res. 2025;30(1):453. doi: 10.1186/s40001-025-02664-7. DOI: https://doi.org/10.1186/s40001-025-02664-7
Ferrell PB, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9(10):1543-1546. doi: 10.2217/14622416.9.10.1543. DOI: https://doi.org/10.2217/14622416.9.10.1543
Park K, Yeich A, Craig T. An unusual presentation of Stevens-Johnson syndrome (SJS) in a patient with two prior SJS episodes. Ann Allergy Asthma Immunol. 2022;129(5):S84. doi: 10.1016/j.anai.2022.08.731. DOI: https://doi.org/10.1016/j.anai.2022.08.731
Gui M, Ni M, Yin X, Zhang T, Li Z. Ibuprofen induced Stevens-Johnson syndrome and liver injury in children: a case report. Transl Pediatr. 2021;10:1737-1742. doi: 10.21037/tp-21-8. DOI: https://doi.org/10.21037/tp-21-8
Kumar N. Stevens-Johnson reaction: A rare case report of ibuprofen-induced hypersensitivity reaction in a young child. J Pediatr Dent. 2021;10(69). doi: 10.14744/JPD.2021.10_69. DOI: https://doi.org/10.14744/JPD.2021.10_69
Güdeloğlu E, Altan V. A reasonable outcome with systemic corticosteroids in a case of ibuprofen induced Stevens-Johnson syndrome. Am J Immunol. 2020;16:27-30. doi: 10.3844/ajisp.2020.27.30 DOI: https://doi.org/10.3844/ajisp.2020.27.30
Abreu S, Castelblanco-Arango I, Gómez-Pineda P, Cardona R. Drug provocation tests to identify analgesic alternatives for an infant with Stevens-Johnson syndrome caused by ibuprofen-acetaminophen. Rev Alerg Mex. 2020;67(2):189-95. doi: 10.29262/ram.v67i2.712. DOI: https://doi.org/10.29262/ram.v67i2.712
Bollampally M, Praneeth G, Prithi A. Ibuprofen induced Steven Johnson syndrome. World J Pharm Med Res. 2017;3(10):165-167.
Jian H, Liyun Z, Gailian Z, Ke X, Ruihong H. Ibuprofen sustained-release capsules induced Stevens-Johnson syndrome. Adverse Drug React J. 2017;19(5):389. doi: 10.3760/cma.j.issn.1008-5734. 2017.05.020.
Angadi S, Karn A. Ibuprofen induced Stevens-Johnson syndrome – toxic epidermal necrolysis in Nepal. Asia Pac Allergy. 2016;6(1):70-73. doi: 10.5415/apallergy.2016.6.1.70. DOI: https://doi.org/10.5415/apallergy.2016.6.1.70
Dobry AS, Himed S, Waters M, Kaffenberger BH. Scoring assessments in Stevens-Johnson syndrome and toxic epidermal necrolysis. Front Med. 2022;9:883121. doi: 10.3389/fmed.2022.883121. DOI: https://doi.org/10.3389/fmed.2022.883121
Torres-Navarro I, Briz-Redón Á, Botella-Estrada R. Accuracy of SCORTEN to predict the prognosis of Stevens-Johnson syndrome/toxic epidermal necrolysis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2020;34(9):2066-2077. doi: 10.1111/jdv.16137. DOI: https://doi.org/10.1111/jdv.16137
Chang HC, Wang TJ, Lin MH, Chen TJ. A review of the systemic treatment of Stevens–Johnson syndrome and toxic epidermal necrolysis. Biomedicines. 2022;10(9):2105. doi: 10.3390/biomedicines10092105. DOI: https://doi.org/10.3390/biomedicines10092105
Noe MH, Micheletti RG. Systemic interventions for treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: summary of a Cochrane review. JAMA Dermatol. 2022;158(12):1436-7. doi: 10.1001/jamadermatol.2022.4543. DOI: https://doi.org/10.1001/jamadermatol.2022.4543
Heuer R, Paulmann M, Mockenhaupt M, Nast A. Systemic immunomodulating therapies for epidermal necrolysis (Stevens-Johnson syndrome/toxic epidermal necrolysis): a systematic review and meta-analysis. J Dtsch Dermatol Ges. 2025;192(1):9-18. doi: 10.1111/ddg.15804. DOI: https://doi.org/10.1111/ddg.15804
Hasegawa A, Abe R. Recent advances in managing and understanding Stevens-Johnson syndrome and toxic epidermal necrolysis. F1000Res. 2020;9:Faculty Rev-1014. doi: 10.12688/f1000research.24748.1. DOI: https://doi.org/10.12688/f1000research.24748.1
Schneider JA, Cohen PR. Stevens-Johnson syndrome and toxic epidermal necrolysis: a concise review with a comprehensive summary of therapeutic interventions emphasizing supportive measures. Adv Ther. 2017;34(6):1235-44. doi: 10.1007/s12325-017-0530-y. DOI: https://doi.org/10.1007/s12325-017-0530-y
Martinez Villarreal JD, Cardenas-de la Garza JA, Ionescu MA, Tatu AL, Busila C, Mokni M, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: A review of current management and innovative therapies. Int J Dermatol. 2025;64(5):650-8. doi: 10.1111/ijd.17768. DOI: https://doi.org/10.1111/ijd.17768
Hsieh MH, Watanabe T, Aihara M. Recent dermatological treatments for Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan. Front Med. 2021;8:636924. doi: 10.3389/fmed.2021.636924. DOI: https://doi.org/10.3389/fmed.2021.636924
Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, Mockenhaupt M. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. 2017;153(6):514-522. doi: 10.1001/jamadermatol.2016.5668. DOI: https://doi.org/10.1001/jamadermatol.2016.5668
Jacobsen A, Olabi B, Langley A, Beecker J, Mutter E, Shelley A, et al. Systemic interventions for treatment of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and SJS/TEN overlap syndrome. Cochrane Database Syst Rev. 2022;(3):CD015050. doi: 10.1002/14651858.CD013130.pub2. DOI: https://doi.org/10.1002/14651858.CD013130.pub2
Hama N, Aoki S, Chen CB, Hasegawa A, Ogawa Y, Vocanson M, et al. Recent progress in Stevens–Johnson syndrome/toxic epidermal necrolysis: diagnostic criteria, pathogenesis and treatment. Br J Dermatol. 2025;192(1):9-18. doi: 10.1093/bjd/ljae321. DOI: https://doi.org/10.1093/bjd/ljae321
Cao J, Zhang X, Xing X, Fan J. Biologic TNF-α inhibitors for Stevens–Johnson Syndrome, toxic epidermal necrolysis, and TEN-SJS overlap: A study-level and patient-level meta-analysis. Dermatol Ther. 2023;13(6):1305-1327. doi: 10.1007/s13555-023-00928-w. DOI: https://doi.org/10.1007/s13555-023-00928-w
Bhandari M, Khullar G. Target and targetoid lesions in dermatology. Indian J Dermatol Venereol Leprol. 2022;88(3):430-434. doi: 10.25259/IJDVL_901_20. DOI: https://doi.org/10.25259/IJDVL_901_20
Pejčić AV. Stevens-Johnson syndrome and toxic epidermal necrolysis associated with the use of macrolide antibiotics: a review of published cases. Int J Dermatol. 2021;60(1):12-24. doi: 10.1111/ijd.15144. DOI: https://doi.org/10.1111/ijd.15144
Tsai TY, Huang IH, Chao YC, Li H, Hsieh TS, Wang HH, et al. Treating toxic epidermal necrolysis with systemic immunomodulating therapies: a systematic review and network meta-analysis. J Am Acad Dermatol. 2021;84(2):390-397. doi: 10.1016/j.jaad.2020.08.122. DOI: https://doi.org/10.1016/j.jaad.2020.08.122
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











