Immunohistochemical Expression of MLH1, PMS2 and P53 in Colorectal Carcinoma with Clinicopathologic Correlation
DOI:
https://doi.org/10.54133/ajms.v9i1.2181Keywords:
Colorectal carcinoma, MLH1, PMS2, p53, Clinicopathologic features, ImmunohistochemistryAbstract
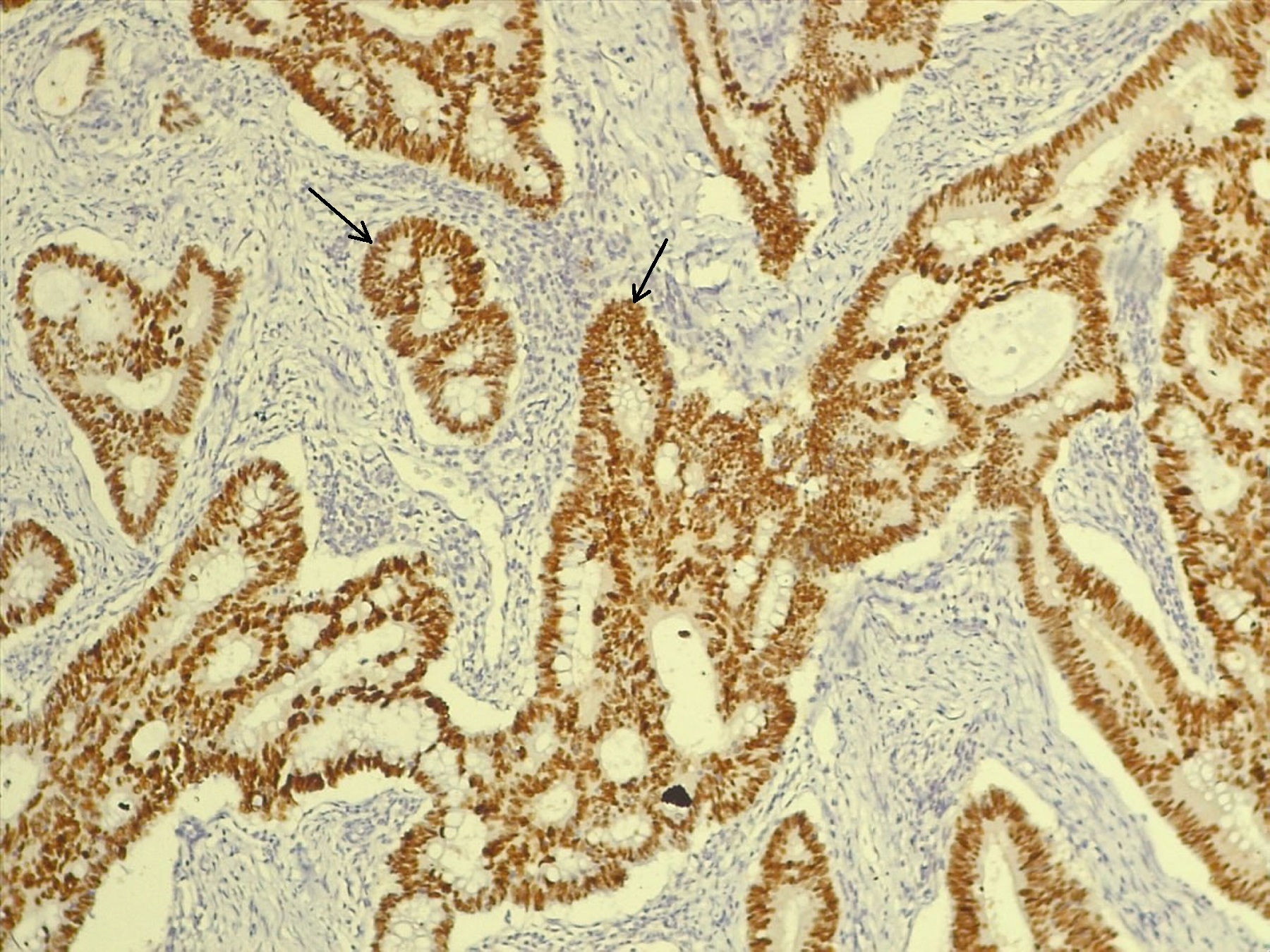
Background: Proteins of the mismatch repair system and p53 are important for prognosis and treatment of colorectal carcinoma. This raises the need for a better definition of clinical criteria that can be used to detect patients who have defects in these proteins. Objective: To detect the correlations between clinicopathologic features and the expressions of MLH1, PMS2, and p53 in colorectal carcinoma. Methods: This is a cross-sectional analytical study. Tissue samples of 102 colorectal carcinomas were collected in the hospitals of Baghdad Medical City. Archived reports of patients provided clinical and pathological data. The study was done during 2023 and 2024. Immunohistochemical staining results for MLH1, PMS2, and p53 proteins were compared to clinicopathologic criteria and to each other. Results: MLH1 loss was more frequent in tumors of the right colon (p=0.019) and tumors with T3 extension (p=0.05). PMS2 absence was predominant in tumors of moderate differentiation (p=0.04), adenocarcinoma, NOS (p=0.05), tumor-free resection margins (p=0.03), and absence of perineural invasion (p=0.04). The wild expression of p53 was more frequent with the absence of lymphovascular tumor invasion (p=0.04). Aberrant p53 is associated with resection margins clear of tumor invasion (p=0.03), adenocarcinoma, NOS (p=0.05), and grade 2 differentiated tumors (p=0.04). PMS2 is associated with MLH1 (p=0.0001). p53 is associated with PMS2 (p=0.04). Conclusions: A number of CRC clinicopathological variables are related to MLH1, PMS2, and p53 expression status. p53 is correlated with PMS2 status. Consequently, p53 may affect the prognosis of CRC with normal PMS2.
Downloads
References
Hossain S, Karuniawati H, Jairoun AA, Urbi Z, Ooi DJ, John A, et al. Colorectal Cancer: A review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers. 2022;14(7):1732. doi: 10.3390/cancers14071732. DOI: https://doi.org/10.3390/cancers14071732
Annual Report, Iraqi Cancer Registry 2022. Iraqi Cancer Board, Ministry of Health, Republic of Iraq, 2022.
Abbas MM, Alkhazrajy LA. Knowledge, attitudes and practices regarding the screening of colorectal cancer among primary care physicians in Baghdad during 2022. J Fac Med Baghdad. 2024;66(1):45–50. doi: 10.32007/jfacmedbagdad.2162. DOI: https://doi.org/10.32007/jfacmedbagdad.2162
Shakir A, Al-Rubaiawi H, Haba MK, Raouf L, Al-Hadad A. Evaluation of CEA,CA19-9, and CA242 tumor markers in patients with colorectal cancer by ELISA technique. Indian J Forensic Med Toxicol. 2020. doi: 10.37506/ijfmt.v14i2.2809. DOI: https://doi.org/10.37506/ijfmt.v14i2.2809
Farhad RM, Saleh ES, Alsammarraie AZ. Clinicopathological features of colorectal cancer in the Iraqi population focusing on age and early-onset of malignancy: A descriptive cross-sectional study. Al-Rafidain J Med Sci. 2023;5:86–91. doi: 10.54133/ajms.v5i.158. DOI: https://doi.org/10.54133/ajms.v5i.158
Alezzi MJ, Alani KH, Alezzi JI. Epidemiology of colorectal polyps in Iraqi patients. Med J Babylon. 2024;21(3):506–510. doi: 10.4103/mjbl.mjbl_348_22. DOI: https://doi.org/10.4103/MJBL.MJBL_348_22
Abdulhassan BA, Mahdi QA, Meeshal MT, Ali MH, Kadhim SMA, Noori A. Descriptive study of patients referred for colonoscopy at gastro-enterology unit at Al-Imamain Al-Kadhemain Medical City in Baghdad. J Fac Med Baghdad. 2024;66(1):11–17. doi: 10.32007/jfacmedbagdad.6612163. DOI: https://doi.org/10.32007/jfacmedbagdad.6612163
Bresam S. Phenotypic and prevalence correlation with recurrent KRAS G12C, G13D, and G22E mutations in colorectal adenocarcinoma. Egypt J Basic Appl Sci.. 2024;11(1):616–625. doi: 10.1080/2314808x.2024.2375091. DOI: https://doi.org/10.1080/2314808X.2024.2375091
Kadhem Mallakh M, Mohammed Mahmood M, Hasan Mohammed Ali S. Immunomolecular investigation of human papillomavirus genotypes (16, 18) and P63 expression in patients with malignant and non-malignant colorectal tumors. Arch Razi Inst. 2022;77(1):383-390. doi: 10.22092/ARI.2021.356608.1879.
Farhad R, Saleh E, Alsammarraie A. No evidence of relationship between colorectal cancer susceptibility and ERCC2 gene polymorphisms. J Adv Biotechnol Exp Ther. 2023;6(3):638. doi: 10.5455/jabet.2023.d155. DOI: https://doi.org/10.5455/jabet.2023.d155
Falih Soliman N, Jasim Mohamad B. Clinical and histopathological characteristics of colorectal cancer in Iraq between 2015-2021. Arch Razi Inst. 2022;77(6):2407-2413. doi: 10.22092/ARI.2022.358613.2263.
Khlf GK, Alash SA. Study the bacterial activity isolated from colon and rectal cancer biopsy in cell lines culture. Biomed Pharmacol J. 2023;16(2):1245–1255. doi: 10.13005/bpj/2705. DOI: https://doi.org/10.13005/10.13005/bpj/2705
Nádorvári ML, Lotz G, Kulka J, Kiss A, Tímár J. Microsatellite instability and mismatch repair protein deficiency: equal predictive markers? Pathol Oncol Res. 2024;30. doi: 10.3389/pore.2024.1611719. DOI: https://doi.org/10.3389/pore.2024.1611719
Bateman AC. DNA mismatch repair proteins: scientific update and practical guide. J Clin Pathol. 2021;74(4):264–268. doi: 10.1136/jclinpath-2020-207281. DOI: https://doi.org/10.1136/jclinpath-2020-207281
Salman AM, Babaei E, Al-Khafaji ASK. Exploring the modulation of MLH1 and MSH2 gene expression in hesperetin-treated breast cancer cells (BT-474). J Adv Pharm Technol Amp Res. 2024;15(1):43–48. doi: 10.4103/japtr.japtr_279_23. DOI: https://doi.org/10.4103/japtr.japtr_279_23
Qaddoori YB, Al-Khafaji ASK, Khashman BM, Abdulghafour KH. The potential role of HDAC1 and HDAC3 immunoexpression in P53 downregulation and tumor aggressiveness of colon and rectum carcinomas patients. Exp Oncol. 2025;46(4):393–401. doi: 10.15407/exp-oncology.2024.04.393. DOI: https://doi.org/10.15407/exp-oncology.2024.04.393
Kim KM, Ahn AR, Park HS, Jang KY, Moon WS, Kang MJ, et al. Clinical significance of p53 protein expression and TP53 variation status in colorectal cancer. BMC Cancer. 2022;22(1). doi: 10.1186/s12885-022-10039-y. DOI: https://doi.org/10.1186/s12885-022-10039-y
Yahya A, Alhamadani ZR, Mundher M. Immunohistochemical expression of retinoblastoma gene product and p53 protein in transitional cell carcinoma of the urinary bladder and its relationship to different clinicopathological parameters. Open Access Macedonian J Med Sci. 2021;9(A):595–609. doi: 10.3889/oamjms.2021.6559. DOI: https://doi.org/10.3889/oamjms.2021.6559
Chen K, Collins G, Wang H, Toh JWT. Pathological features and prognostication in colorectal cancer. Curr Oncol. 2021;28(6):5356–5383. doi: 10.3390/curroncol28060447. DOI: https://doi.org/10.3390/curroncol28060447
Chen W, Frankel WL. A practical guide to biomarkers for the evaluation of colorectal cancer. Modern Pathol. 2018;32:1–15. doi: 10.1038/s41379-018-0136-1. DOI: https://doi.org/10.1038/s41379-018-0136-1
Osakabe M, Yamada N, Sugimoto R, Uesugi N, Nakao E, Honda M, et al. The pattern-based interpretation of p53 immunohistochemical expression as a surrogate marker for TP53 mutations in colorectal cancer. Virchows Arch. 2024; doi: 10.1007/s00428-024-03790-z. DOI: https://doi.org/10.1007/s00428-024-03790-z
Fan WX, Su F, Zhang Y, Zhang XL, Du YY, Gao YJ, et al. Oncological characteristics, treatments and prognostic outcomes in MMR-deficient colorectal cancer. Biomarker Res. 2024;12(1). doi: 10.1186/s40364-024-00640-7. DOI: https://doi.org/10.1186/s40364-024-00640-7
Mei WJ, Mi M, Qian J, Xiao N, Yuan Y, Ding PR. Clinicopathological characteristics of high microsatellite instability/mismatch repair-deficient colorectal cancer: A narrative review. Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.1019582. DOI: https://doi.org/10.3389/fimmu.2022.1019582
Nádorvári ML, Kenessey I, Kiss A, Barbai T, Kulka J, Rásó E, et al. Comparison of standard mismatch repair deficiency and microsatellite instability tests in a large cancer series. J Transl Med. 2024;22(1). doi: 10.1186/s12967-024-04960-y. DOI: https://doi.org/10.1186/s12967-024-04960-y
Deng Z, Luo Y, Chen X, Pan T, Rui Y, Hu H, et al. Pathological response following neoadjuvant immunotherapy and imaging characteristics in dMMR/MSI-H locally advanced colorectal cancer. Front Immunol. 2024;15. doi: 10.3389/fimmu.2024.1466497. DOI: https://doi.org/10.3389/fimmu.2024.1466497
Guan J, Li GM. DNA mismatch repair in cancer immunotherapy. NAR Cancer [Internet]. 2023 Jun 8;5(3). Available from: https://doi.org/10.1093/narcan/zcad031 DOI: https://doi.org/10.1093/narcan/zcad031
Lu Z, Shen L, Zhang H, Jiao X, Wang Y, Chen H, et al. Effect of TP53 mutation on antitumor immunity and responsiveness to immunotherapy in colorectal cancer. J Clin Oncol. 2020;38(15_suppl):e16014. doi: 10.1200/jco.2020.38.15_suppl.e16014. DOI: https://doi.org/10.1200/JCO.2020.38.15_suppl.e16014
Ye M, Ru G, Yuan H, Qian L, He X, Li S. Concordance between microsatellite instability and mismatch repair protein expression in colorectal cancer and their clinicopathological characteristics: a retrospective analysis of 502 cases. Front Oncol. 2023;13. doi: 10.3389/fonc.2023.1178772. DOI: https://doi.org/10.3389/fonc.2023.1178772
White A, Ironmonger L, Steele RJC, Ormiston-Smith N, Crawford C, Seims A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer. 2018;18(1). https://doi.org/10.1186/s12885-018-4786-7 DOI: https://doi.org/10.1186/s12885-018-4786-7
Hashmi AA, Bukhari U, Rizwan R, Faisal F, Kumar R, Malik UA, et al. Mismatch repair deficient (DMMR) colorectal carcinoma in a Pakistani cohort: association with clinical and pathological parameters. Cureus. 2023. doi: 10.7759/cureus.42781. DOI: https://doi.org/10.7759/cureus.42781
Jiang W, Sui QQ, Li WL, Ke CF, Ling YH, Liao LE, et al. Low prevalence of mismatch repair deficiency in Chinese colorectal cancers: a multicenter study. Gastroenterol Rep. 2020;8(5):399–403. doi: 10.1093/gastro/goaa006. DOI: https://doi.org/10.1093/gastro/goaa006
Zeng Z, Yan Q, Chen G, Zhang X, Huang J, Fu K, et al. Characteristics of colorectal carcinoma patients with PMS2 defects detected by immunohistochemistry. Eur J Cancer Prevent. 2020;30(3):251–257. doi: 10.1097/cej.0000000000000620. DOI: https://doi.org/10.1097/CEJ.0000000000000620
Cao DZ, Ou XL, Yu T. The association of p53 expression levels with clinicopathological features and prognosis of patients with colon cancer following surgery. Oncol Lett. 2017;13(5):3538–3546. doi: 10.3892/ol.2017.5929. DOI: https://doi.org/10.3892/ol.2017.5929
Zarbaliyev E, Turhan N, Çelik S, Çağlıkülekçi M. Lymphovascular invasion in colorectal cancers: can we predict it preoperatively? Ann Coloproctol. 2024;40(3):245–252. doi: 10.3393/ac.2023.00458.0065. DOI: https://doi.org/10.3393/ac.2023.00458.0065
Kataoka M, Hirano Y, Ishii T, Kondo H, Asari M, Ishikawa S, et al. Impact of lymphovascular invasion in patients with Stage II colorectal cancer: a propensity score-matched study. In Vivo. 2021;35(1):525–531. doi: 10.21873/invivo.12287. DOI: https://doi.org/10.21873/invivo.12287
Oh HJ, Bae JM, Wen X, Jung S, Kim Y, Kim KJ, et al. p53 expression status is associated with cancer-specific survival in stage III and high-risk stage II colorectal cancer patients treated with oxaliplatin-based adjuvant chemotherapy. Br J Cancer. 2019;120(8):797–805. doi: 10.1038/s41416-019-0429-2. DOI: https://doi.org/10.1038/s41416-019-0429-2
Lin Z, Zheng Y, Yang J, Jin W, Wang J, Wang W, et al. Prognostic analysis of lymphovascular invasion in stages I–III colorectal cancer. Am J Clin Oncol. 2023;46(8):366–373. doi: 10.1097/coc.0000000000001015. DOI: https://doi.org/10.1097/COC.0000000000001015
Smith HG, Schlesinger NH, Qvortrup C, Chiranth D, Lundon D, Ben-Yaacov A, et al. Variations in the definition and perceived importance of positive resection margins in patients with colorectal cancer – an EYSAC international survey. Eur J Surg Oncology. 2023;49(11):107072. doi: 10.1016/j.ejso.2023.107072. DOI: https://doi.org/10.1016/j.ejso.2023.107072
Oh HH, Kim JS, Lim JW, Lim CJ, Seo YE, You GR, et al. Clinical outcomes of colorectal neoplasm with positive resection margin after endoscopic submucosal dissection. Sci Rep. 2024;14(1). doi: 10.1038/s41598-024-63129-1. DOI: https://doi.org/10.1038/s41598-024-63129-1
Balbaa MA, Elkady N, Abdelrahman EM. Predictive factors of positive circumferential and longitudinal margins in early T3 colorectal cancer resection. Int J Surg Oncol. 2020;2020:1–8. doi: 10.1155/2020/6789709. DOI: https://doi.org/10.1155/2020/6789709
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











