Molecular Mechanism of Physical Exercise in Increasing Genetic Expression of Sirtuin 1 (SIRT1): A Systematic Review
DOI:
https://doi.org/10.54133/ajms.v9i2.2148Keywords:
Molecular mechanisms, Oxidative stress, Physical exercise, SIRT1Abstract
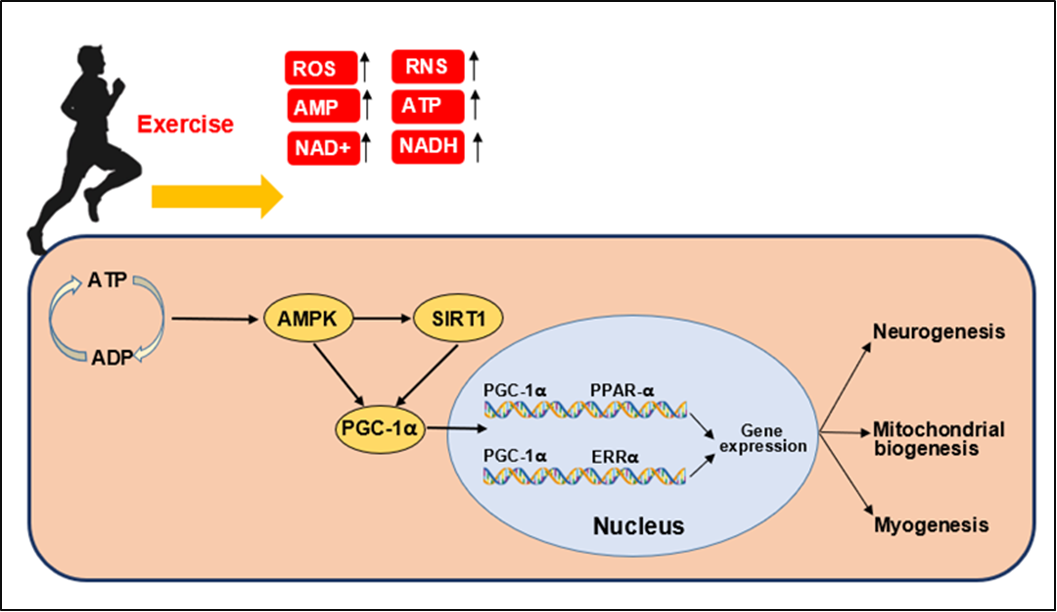
Background: Obesity is associated with decreased levels of protein sirtuin 1 (SIRT1). This decrease can disrupt SIRT1 activity, leading to reduced antioxidant defenses, increased oxidative stress, impaired fatty acid oxidation, and reduced energy expenditure. These changes can contribute to a positive energy balance and long-term weight gain. Exercise is an alternative way to prevent decreased sirtuin 1 level. Sirtuin 1 plays a role in cell maintenance, stress markers, determining cell fate, maintaining energy balance, preventing DNA damage, and maintaining the stability and continuity of cell function. Objective: This study aims to determine and measure how the mechanism of physical exercise can increase the expression of SIRT1. Methods: Several journal databases, such as Embase, PubMed, Web of Science, ScienceDirect, and Scopus, were searched for this study. The criteria for this study included papers on oxidative stress, physical exercise, and SIRT1 published during the previous five years. The only publications rejected for inclusion in this analysis were those published in non-reputable journals. Using Embase, Web of Science, PubMed, ScienceDirect, and Scopus databases, a total of 1,891 publications were found. These systemic breakthrough criteria have been examined and discussed in about 9 studies. Results: Physical exercise can increase the expression of SIRT1. Conclusions: Physical exercise is proven to increase the expression of SIRT1 to influence the increase of biogenesis in mitochondria, which will have a favorable and beneficial impact on the degree of human health.
Downloads
References
Alves-Fernandes DK, Jasiulionis MG. The role of SIRT1 on DNA damage response and epigenetic alterations in cancer. Int J Mol Sci. 2019;20(13):3153. doi: 10.3390/ijms20133153. DOI: https://doi.org/10.3390/ijms20133153
Aird TP, Farquharson AJ, Bermingham KM, O'Sulllivan A, Drew JE, Carson BP. Divergent serum metabolomic, skeletal muscle signaling, transcriptomic, and performance adaptations to fasted versus whey protein-fed sprint interval training. Am J Physiol Endocrinol Metab. 2021;321(6):E802-E820. doi: 10.1152/ajpendo.00265.2021. DOI: https://doi.org/10.1152/ajpendo.00265.2021
Juan CG, Matchett KB, Davison GW. A systematic review and meta-analysis of the SIRT1 response to exercise. Sci Rep. 2023;13(1):14752. doi: 10.1038/s41598-023-38843-x. DOI: https://doi.org/10.1038/s41598-023-38843-x
Yang Y, Liu Y, Wang Y, Chao Y, Zhang J, Jia Y, et al. Regulation of SIRT1 and its roles in inflammation. Front Immunol. 2022;13:831168. doi: 10.3389/fimmu.2022.831168. DOI: https://doi.org/10.3389/fimmu.2022.831168
Musilanga N, Nasib H, Jackson G, Shayo F, Nhanga C, Girukwigomba S, et al. Exploring the prevalence and components of metabolic syndrome in Sub-Saharan African type 2 diabetes mellitus patients: A systematic review and meta-analysis. J Obes. 2024;2024:1240457. doi: 10.1155/2024/1240457. DOI: https://doi.org/10.21203/rs.3.rs-3958331/v1
Hamid S, Groot W, Pavlova M. Trends in cardiovascular diseases and associated risks in sub-Saharan Africa: a review of the evidence for Ghana, Nigeria, South Africa, Sudan and Tanzania. Aging Male. 2019;22(3):169-176. doi: 10.1080/13685538.2019.1582621. DOI: https://doi.org/10.1080/13685538.2019.1582621
Amaeshi L, Kalejaiye OO, Olopade OB, Kehinde M. Relationship between hematologic inflammatory markers and glycemic control in type 2 diabetes mellitus patients in a tertiary hospital in Nigeria. Cureus. 2024;16(8):e68186. doi: 10.7759/cureus.68186. DOI: https://doi.org/10.7759/cureus.68186
Holmannova D, Borsky P, Andrys C, Kremlacek J, Fiala Z, Parova H, et al. The influence of metabolic syndrome on potential aging biomarkers in participants with metabolic syndrome compared to healthy controls. Biomedicines. 2024;12(1):242. doi: 10.3390/biomedicines12010242. DOI: https://doi.org/10.3390/biomedicines12010242
Chandrasekaran P, Weiskirchen R. The role of obesity in type 2 diabetes mellitus-an overview. Int J Mol Sci. 2024;25(3):1882. doi: 10.3390/ijms25031882. DOI: https://doi.org/10.3390/ijms25031882
Klein S, Gastaldelli A, Yki-Järvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab. 2022;34(1):11-20. doi: 10.1016/j.cmet.2021.12.012. DOI: https://doi.org/10.1016/j.cmet.2021.12.012
Li S, Wang P, Wang J, Zhao J, Wang X, Liu T. Effect of mind-body exercise on risk factors for metabolic syndrome including insulin resistance: a meta-analysis. Front Endocrinol (Lausanne). 2024;15:1289254. doi: 10.3389/fendo.2024.1289254. DOI: https://doi.org/10.3389/fendo.2024.1289254
Vargas-Ortiz K, Pérez-Vázquez V, Macías-Cervantes MH. Exercise and sirtuins: A way to mitochondrial health in skeletal muscle. Int J Mol Sci. 2019;20(11):2717. doi: 10.3390/ijms20112717. DOI: https://doi.org/10.3390/ijms20112717
Wang F, Wang X, Liu Y, Zhang Z. Effects of exercise-induced ROS on the pathophysiological functions of skeletal muscle. Oxid Med Cell Longev. 2021;2021:3846122. doi: 10.1155/2021/3846122. DOI: https://doi.org/10.1155/2021/3846122
Sawada Y, Ichikawa H, Ebine N, Minamiyama Y, Alharbi AAD, Iwamoto N, et al. Effects of high-intensity anaerobic exercise on the scavenging activity of various reactive oxygen species and free radicals in athletes. Nutrients. 2023;15(1):222. doi: 10.3390/nu15010222. DOI: https://doi.org/10.3390/nu15010222
Yeh YK, Yen FS, Hwu CM. Diet and exercise are a fundamental part of comprehensive care for type 2 diabetes. J Diabetes Investig. 2023;14(8):936-939. doi: 10.1111/jdi.14043. DOI: https://doi.org/10.1111/jdi.14043
Rohnejad B, Monazzami A. Effects of high-intensity intermittent training on some inflammatory and muscle damage indices in overweight middle-aged men. Apunt Sport Med. 2023;58:100404. doi: 10.1016/j.apunsm.2023.100404. DOI: https://doi.org/10.1016/j.apunsm.2023.100404
Shamsnia E, Matinhomaee H, Azarbayjani MA, Peeri M, The effect of aerobic exercise on oxidative stress in skeletal muscle tissue: A narrative review. Gene Cell Tissue. 2023;10(4):e131964. doi: 10.5812/gct-131964. DOI: https://doi.org/10.5812/gct-131964
Suzuki K, Tominaga T, Ruhee RT, Ma S. Characterization and modulation of systemic inflammatory response to exhaustive exercise in relation to oxidative stress. Antioxidants (Basel). 2020;9(5):401. doi: 10.3390/antiox9050401. DOI: https://doi.org/10.3390/antiox9050401
Thirupathi A, Pinho RA, Ugbolue UC, He Y, Meng Y, Gu Y. Effect of running exercise on oxidative stress biomarkers: A systematic review. Front Physiol. 2021;11:610112. doi: 10.3389/fphys.2020.610112. DOI: https://doi.org/10.3389/fphys.2020.610112
Sohouli MH, Eslamian G, Rohani P, Zand H, Guimarães NS. The effect of weight loss therapies on sirtuin 1 regulation: a systematic review and meta-analysis of randomized controlled trials. BMC Nutr. 2024;10(1):111. doi: 10.1186/s40795-024-00921-2. DOI: https://doi.org/10.1186/s40795-024-00921-2
Cho SY, Chung YS, Yoon HK, Roh HT. Impact of exercise intensity on systemic oxidative stress, inflammatory responses, and sirtuin levels in healthy male volunteers. Int J Environ Res Public Health. 2022;19(18):11292. doi: 10.3390/ijerph191811292. DOI: https://doi.org/10.3390/ijerph191811292
Ghasemi E, Afzalpour ME, Nayebifar S. Combined high-intensity interval training and green tea supplementation enhance metabolic and antioxidant status in response to acute exercise in overweight women. J Physiol Sci. 2020;70(1):31. doi: 10.1186/s12576-020-00756-z. DOI: https://doi.org/10.1186/s12576-020-00756-z
Potthast AB, Nebl J, Wasserfurth P, Haufe S, Eigendorf J, Hahn A, et al. Impact of nutrition on short-term exercise-induced sirtuin regulation: Vegans differ from omnivores and Lacto-Ovo vegetarians. Nutrients. 2020;12(4):1004. doi: 10.3390/nu12041004. DOI: https://doi.org/10.3390/nu12041004
Amirsasan R, Dolgari R, Vakili J, Effects of Pilates training and turmeric supplementation on sirtuin 1 level and body composition in postmenopausal females with sedentary overweight: A randomized, double-blind, clinical trial. Zahedan J Res Med Sci. 2019;21(3):e81620. doi: 10.5812/zjrms.81620. DOI: https://doi.org/10.5812/zjrms.81620
Wasserfurth P, Nebl J, Rühling MR, Shammas H, Bednarczyk J, Koehler K, et al. Impact of dietary modifications on plasma sirtuins 1, 3 and 5 in older overweight individuals undergoing 12-weeks of circuit training. Nutrients. 2021;13(11):3824. doi: 10.3390/nu13113824. DOI: https://doi.org/10.3390/nu13113824
Mohammed A, Sadek NB, Mohammed MM, Khowailed EA, Rashed LA. Interplay between exercise and SIRT1 in skeletal muscle of diabetic male rats. Med J Cairo Univ. 2019;87:3573-3579. doi: 10.21608/mjcu.2019.69894. DOI: https://doi.org/10.21608/mjcu.2019.69894
Shi D, Hao Z, Qi W, Jiang F, Liu K, Shi X. Aerobic exercise combined with chlorogenic acid exerts neuroprotective effects and reverses cognitive decline in Alzheimer's disease model mice (APP/PS1) via the SIRT1/ /PGC-1α/PPARγ signaling pathway. Front Aging Neurosci. 2023;15:1269952. doi: 10.3389/fnagi.2023.1269952. DOI: https://doi.org/10.3389/fnagi.2023.1269952
Baskaran R, Balasubramanian B, Ho JH, Wang MF, Abomughaid MM, Yang HS, et al. VH-4-A bioactive peptide from soybean and exercise training constrict hypertension in rats through activating cell survival and AMPKα1, Sirt1, PGC1α, and FoX3α. Molecules. 2022;27(22):7705. doi: 10.3390/molecules27227705. DOI: https://doi.org/10.3390/molecules27227705
Lu Z, Xu Y, Song Y, Bíró I, Gu Y. A mixed comparisons of different intensities and types of physical exercise in patients with diseases related to oxidative stress: A systematic review and network meta-analysis. Front Physiol. 2021;12:700055. doi: 10.3389/fphys.2021.700055. DOI: https://doi.org/10.3389/fphys.2021.700055
Kawamura T, Higashida K, Muraoka I. Application of molecular hydrogen as a novel antioxidant in sports science. Oxid Med Cell Longev. 2020;2020:2328768. doi: 10.1155/2020/2328768. DOI: https://doi.org/10.1155/2020/2328768
Sylviana N, Natalia C, Goenawan H, Pratiwi YS, Setiawan I, Tarawan VM, et al. Effect of short-term endurance exercise on COX IV and PGC-1 mRNA expression levels in rat skeletal muscle. Biomed Pharmacol J. 2019;12(3). doi: 10.13005/bpj/1759. DOI: https://doi.org/10.13005/bpj/1759
van der Zwaard S, Brocherie F, Jaspers RT. Under the hood: Skeletal muscle determinants of endurance performance. Front Sports Act Living. 2021;3:719434. doi: 10.3389/fspor.2021.719434. DOI: https://doi.org/10.3389/fspor.2021.719434
Irawati NAV, Sylviana N, Lubis L. Role of exercise intensity in skeletal muscle hypertrophy. J Biomedika dan Kesehat. 2024;7(1):124-132. DOI: https://doi.org/10.18051/JBiomedKes.2024.v7.124-132
Mcleod JC, Currier BS, Lowisz CV, Phillips SM. The influence of resistance exercise training prescription variables on skeletal muscle mass, strength, and physical function in healthy adults: An umbrella review. J Sport Health Sci. 2024;13(1):47-60. doi: 10.1016/j.jshs.2023.06.005. DOI: https://doi.org/10.1016/j.jshs.2023.06.005
Cordiano R, Di Gioacchino M, Mangifesta R, Panzera C, Gangemi S, Minciullo PL. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: An update. Molecules. 2023;28(16):5979. doi: 10.3390/molecules28165979. DOI: https://doi.org/10.3390/molecules28165979
Souissi W, Bouzid MA, Farjallah MA, Ben Mahmoud L, Boudaya M, Engel FA, et al. Effect of different running exercise modalities on post-exercise oxidative stress markers in trained athletes. Int J Environ Res Public Health. 2020;17(10):3729. doi: 10.3390/ijerph17103729. DOI: https://doi.org/10.3390/ijerph17103729
Ye Y, Lin H, Wan M, Qiu P, Xia R, He J, et al. The effects of aerobic exercise on oxidative stress in older adults: A systematic review and meta-analysis. Front Physiol. 2021;12:701151. doi: 10.3389/fphys.2021.701151. DOI: https://doi.org/10.3389/fphys.2021.701151
Kazemi SS, Heidarianpour A, Shokri E. Effect of resistance training and high-intensity interval training on metabolic parameters and serum level of Sirtuin1 in postmenopausal women with metabolic syndrome: a randomized controlled trial. Lipids Health Dis. 2023;22(1):177. doi: 10.1186/s12944-023-01940-x. DOI: https://doi.org/10.1186/s12944-023-01940-x
Tagliari CFDS, de Oliveira CN, Vogel GM, da Silva PB, Linden R, Lazzaretti RK, et al. Investigation of SIRT1 gene variants in HIV-associated lipodystrophy and metabolic syndrome. Genet Mol Biol. 2020;43(1):e20190142. doi: 10.1590/1678-4685-GMB-2019-0142. DOI: https://doi.org/10.1590/1678-4685-gmb-2019-0142
Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11(1):139-150. doi: 10.1089/rej.2007.0576. DOI: https://doi.org/10.1089/rej.2007.0576
Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid Redox Signal. 2013;18(10):1208-1246. doi:10.1089/ars.2011.4498. DOI: https://doi.org/10.1089/ars.2011.4498
Jia D, Hou L, Lv Y, Xi L, Tian Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1α/PI3K/Akt signaling. J Cell Physiol. 2019;234(12):23705-23718. doi: 10.1002/jcp.28939. DOI: https://doi.org/10.1002/jcp.28939
Ma JK, Scribbans T, Edgett B, Colin Boyd JA, Simpson CP, Little J, et al. Extremely low-volume, high-intensity interval training improves exercise capacity and increases mitochondrial protein content in human skeletal muscle. Open J Mol Integr Physiol. 2013;3:202. doi: 10.4236/ojmip.2013.34027. DOI: https://doi.org/10.4236/ojmip.2013.34027
Marton O, Koltai E, Nyakas C, Bakonyi T, Zenteno-Savin T, Kumagai S, et al. Aging and exercise affect the level of protein acetylation and SIRT1 activity in cerebellum of male rats. Biogerontology. 2010;11(6):679-686. doi: 10.1007/s10522-010-9279-2. DOI: https://doi.org/10.1007/s10522-010-9279-2
Masuki S, Morikawa M, Nose H. High-intensity walking time is a key determinant to increase physical fitness and improve health outcomes after interval walking training in middle-aged and older people. Mayo Clin Proc. 2019;94(12):2415-2426. doi: 10.1016/j.mayocp.2019.04.039. DOI: https://doi.org/10.1016/j.mayocp.2019.04.039
Tsilogianni Z, Baker JR, Papaporfyriou A, Papaioannou AI, Papathanasiou E, Koulouris NG, et al. Sirtuin 1: Endocan and sestrin 2 in different biological samples in patients with asthma. Does severity make the difference? J Clin Med. 2020;9(2):473. doi: 10.3390/jcm9020473. DOI: https://doi.org/10.3390/jcm9020473
Ghasemi E, Nayebifar S. Benefits of 10 weeks of high-intensity interval training and green tea supplementation on cardiovascular risk factors and VO2max in overweight women. J Res Med Sci. 2019;24:79. doi: 10.4103/jrms.JRMS_499_18. DOI: https://doi.org/10.4103/jrms.JRMS_499_18
Anderson G, Rodriguez M, Reiter RJ. Multiple sclerosis: Melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int J Mol Sci. 2019;20(21):5500. doi: 10.3390/ijms20215500. DOI: https://doi.org/10.3390/ijms20215500
Nebl J, Drabert K, Haufe S, Wasserfurth P, Eigendorf J, Tegtbur U, et al. Exercise-induced oxidative stress, nitric oxide and plasma amino acid profile in recreational runners with vegetarian and non-vegetarian dietary patterns. Nutrients. 2019;11(8):1875. doi: 10.3390/nu11081875. DOI: https://doi.org/10.3390/nu11081875
Lieberman DE, Kistner TM, Richard D, Lee IM, Baggish AL. The active grandparent hypothesis: Physical activity and the evolution of extended human healthspans and lifespans. Proc Natl Acad Sci U S A. 2021;118(50):e2107621118. doi: 10.1073/pnas.2107621118. DOI: https://doi.org/10.1073/pnas.2107621118
Ferrara L, Joksimovic M, D’Angelo S. Modulation of mitochondrial biogenesis: Action of physical activity and phytochemicals. J Phys Educ Sport. 2021;21(1):425-433. doi: 10.7752/jpes.2021.01042. DOI: https://doi.org/10.7752/jpes.2021.01042
Tokumitsu H, Sakagami H. Molecular mechanisms underlying Ca2+/Calmodulin-dependent protein kinase kinase signal transduction. Int J Mol Sci. 2022;23(19):11025. doi: 10.3390/ijms231911025. DOI: https://doi.org/10.3390/ijms231911025
Jevtovic F. Combination of metformin and exercise in management of metabolic abnormalities observed in type 2 diabetes mellitus. Diabetes, Metab Syndr Obes. 2021;14:4043-4057. doi: 10.2147/DMSO.S328694. DOI: https://doi.org/10.2147/DMSO.S328694
Han Y, Liu Y, Zhang Y, Wang W, Lv T, Huang J, Peng X. The Role and Application of the AMPK-Sirtuins Network in Cellular Senescence. Front Biosci (Landmark Ed). 2023;28(10):250. doi: 10.31083/j.fbl2810250. DOI: https://doi.org/10.31083/j.fbl2810250
Ma S, Sun S, Geng L, Song M, Wang W, Ye Y, et al. Caloric restriction reprograms the single-cell transcriptional landscape of Rattus norvegicus aging. Cell. 2020;180(5):984-1001. doi: 10.1016/j.cell.2020.02.008. DOI: https://doi.org/10.1016/j.cell.2020.02.008
Ulbricht ASSF, de Lima DD, Werlang-Coelho C, Magro DD, Donat B, Vieira MR, et al. Effects of aerobic exercise training on oxidative stress in the skeletal muscles of obese rats. Rev Bras Med do Esporte. 2019;25(5):404-408. doi:10.1590/1517-869220192505184278. DOI: https://doi.org/10.1590/1517-869220192505184278
Abu Shelbayeh O, Arroum T, Morris S, Busch KB. PGC-1α is a master regulator of mitochondrial lifecycle and ROS stress response. Antioxidants (Basel). 2023;12(5):1075. doi: 10.3390/antiox12051075. DOI: https://doi.org/10.3390/antiox12051075
Aggarwal R, Potel KN, McFalls EO, Butterick TA, Kelly RF. Novel therapeutic approaches enhance PGC1-alpha to reduce oxidant stress-inflammatory signaling and improve functional recovery in hibernating myocardium. Antioxidants (Basel). 2022;11(11):2155. doi: 10.3390/antiox11112155. DOI: https://doi.org/10.3390/antiox11112155
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











