Correlation Between Tissue EGFR Mutations and Telomerase Activity in Tissue and Blood of Patients with Lung Cancer: Immunohistochemical and Molecular Study
DOI:
https://doi.org/10.54133/ajms.v9i1.2133Keywords:
Epidermal growth factor receptor, Immunohistochemistry and PCR, Non-small cell lung cancer, TelomeraseAbstract
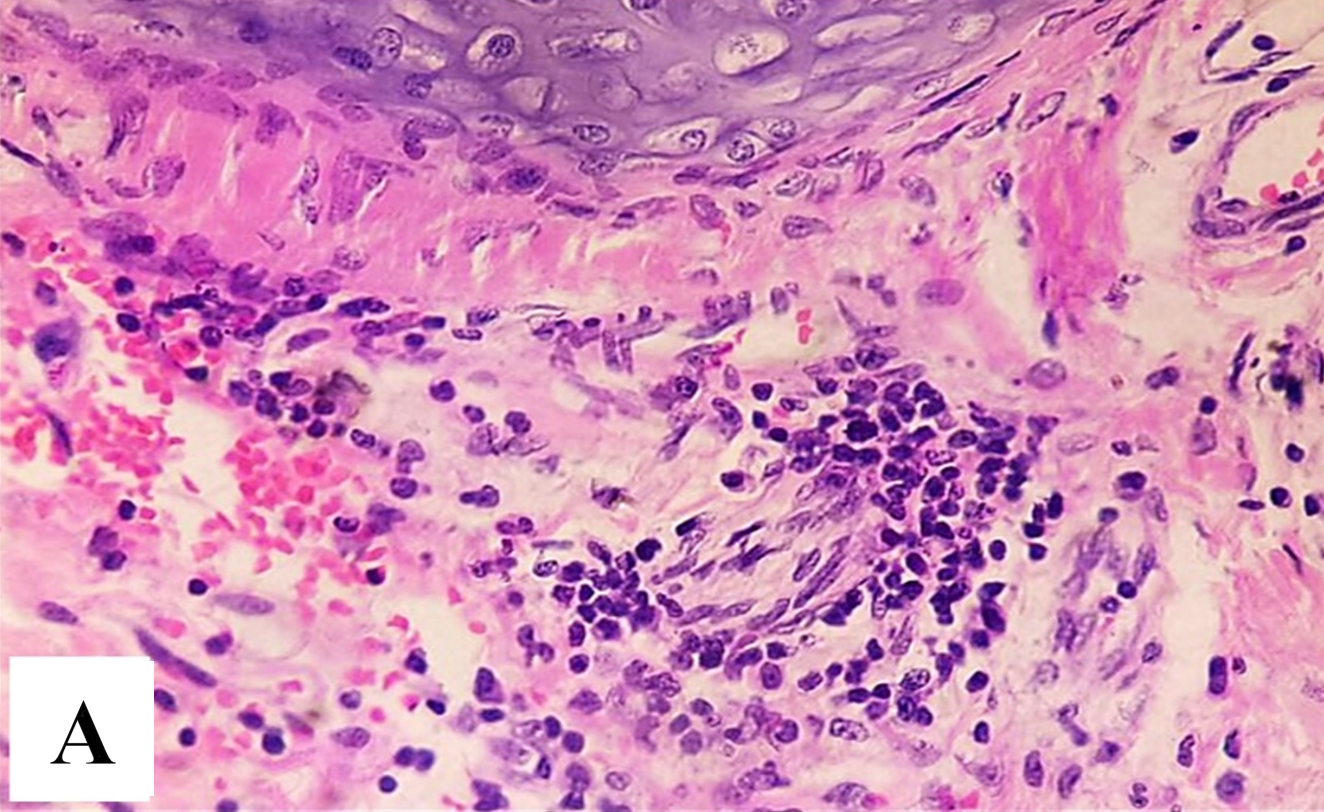
Background: Lung cancer remains one of the leading causes of cancer-related mortality worldwide. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer. Epidermal growth factor receptor (EGFR) mutations are known to drive tumor progression, while telomerase activity is a key mechanism in cancer cell immortality. Objective: This study aims to investigate the correlation between EGFR mutations and telomerase activity in the blood of patients with lung cancer. Methods: This study aims to investigate the correlation between EGFR mutations and telomerase activity in blood and tissue of patients with lung cancer. A cohort study was analyzed for EGFR mutations in tissue and telomerase activity levels in blood using molecular techniques such as polymerase chain reaction (PCR) and in tissue biopsy by using telomerase immunohistochemistry (IHC). Results: The findings suggest a significant correlation between specific EGFR mutations in tissue and increased telomerase activity in blood. Conclusions: This study provides insights into tumor prognosis, response to therapy, and the detection of tumor development.
Downloads
References
Khaddour K, Jonna S, Deneka A, Patel JD, Abazeed ME, Golemis E, et al. Targeting the epidermal growth factor receptor in EGFR-mutated lung cancer: current and emerging therapies. Cancers. 2021;13(13):3164. doi: 10.3390/cancers13133164. DOI: https://doi.org/10.3390/cancers13133164
da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Ann Rev Pathol Mech Dis. 2011;6(1):49-69. doi: 10.1146/annurev-pathol-011110-130206. DOI: https://doi.org/10.1146/annurev-pathol-011110-130206
Yoneda K, Imanishi N, Ichiki Y, Tanaka F. Treatment of non-small cell lung cancer with EGFR-mutations. J UOEH. 2019;41(2):153-163. doi: 10.7888/juoeh.41.153. DOI: https://doi.org/10.7888/juoeh.41.153
Dawood NS, Mussttaf RA, AL-Sahlanee MHR. Model for prediction of the weight and height measurements of patients with disabilities for diagnosis and therapy. Int J Bioautomat. 2021;25(4). doi: 10.54133/ajms.v6i1.410. DOI: https://doi.org/10.7546/ijba.2021.25.4.000824
Raheem HM, Dawood NS, Al-khalisy MH. The role of Modulation Complexity Score (MCS) of the VMAT and IMRT techniques in the treatment planning of left non-small lung cancer. Oncol Radiother. 2023;17(5):137-142.
Noureen N, Wu S, Lv Y, Yang J, Alfred Yung W, Gelfond J, et al. Integrated analysis of telomerase enzymatic activity unravels an association with cancer stemness and proliferation. Nat Commun. 2021;12(1):139. doi: 10.1038/s41467-020-20474-9. DOI: https://doi.org/10.1038/s41467-020-20474-9
Robinson NJ, Schiemann WP. Telomerase in cancer: function, regulation, and clinical translation. Cancers. 2022;14(3):808. doi: 10.3390/cancers14030808. DOI: https://doi.org/10.3390/cancers14030808
Dikmen ZG, Gellert GC, Jackson S, Gryaznov S, Tressler R, Dogan P, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65(17):7866-7873. doi: 10.1158/0008-5472.CAN-05-1215. DOI: https://doi.org/10.1158/0008-5472.CAN-05-1215
Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99(7):1385-1389. doi: 10.1111/j.1349-7006.2008.00831.x. DOI: https://doi.org/10.1111/j.1349-7006.2008.00831.x
Liu XG, Li M, Mai SJ, Cai RJ. Telomere length-related signature as a novel biomarker of prognosis and immune response in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2022;26(4):1304-1319. doi: 10.26355/eurrev_202202_28124.
Augustine T, Maitra R, Goel S. Telomere length regulation through epidermal growth factor receptor signaling in cancer. Genes Cancer. 2017;8(5-6):550. doi: 10.18632/genesandcancer.140. DOI: https://doi.org/10.18632/genesandcancer.140
Chen Z, Vallega KA, Wang D, Quan Z, Fan S, Wang Q, et al. Inhibition of hTERT/telomerase/telomere mediates therapeutic efficacy of osimertinib in EGFR mutant lung cancer. J Exp Med. 2024;221(11):e20240435. doi: 10.1084/jem.20240435. DOI: https://doi.org/10.1084/jem.20240435
Shay JW, Wright WE. Telomerase therapeutics for cancer: challenges and new directions. Nat Rev Drug Discov. 2006;5(7):577-584. doi: 10.1038/nrd2081. DOI: https://doi.org/10.1038/nrd2081
Araki T, Kanda S, Horinouchi H, Ohe Y. Current treatment strategies for EGFR-mutated non-small cell lung cancer: from first line to beyond osimertinib resistance. Jpn J Clin Oncol. 2023;53(7):547-561. doi: 10.1093/jjco/hyad052. DOI: https://doi.org/10.1093/jjco/hyad052
Chen Q, Zheng X, Cheng W, Li J. Landscape of targeted therapies for lung squamous cell carcinoma. Front Oncol. 2024;14:1467898. doi: 10.3389/fonc.2024.1467898. DOI: https://doi.org/10.3389/fonc.2024.1467898
Wei R, Cao L, Pu H, Wang H, Zheng Y, Niu X, et al. TERT polymorphism rs2736100-C is associated with EGFR mutation–positive non–small cell lung cancer. Clin Cancer Res. 2015;21(22):5173-5180. doi: 10.1158/1078-0432.CCR-15-0009. DOI: https://doi.org/10.1158/1078-0432.CCR-15-0009
Goh F, Yang IA, Bowman RV, Fong KM. Subtype variation and actionability of telomere length abnormality in lung cancer. Transl Lung Cancer Res. 2018;7(Suppl 3):S251. doi: 10.21037/tlcr.2018.09.03. DOI: https://doi.org/10.21037/tlcr.2018.09.03
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











