Assessment of mRNA Levels of Tumor Antigen (PRAME) and Clinical Outcomes in Newly Diagnosed Cases of Acute Leukemia
DOI:
https://doi.org/10.54133/ajms.v8i1.1764Keywords:
Acute Leukemia, Cancer Testing Antigen, Monitors Minimal Residual Disease, PRAME Gene ExpressionAbstract
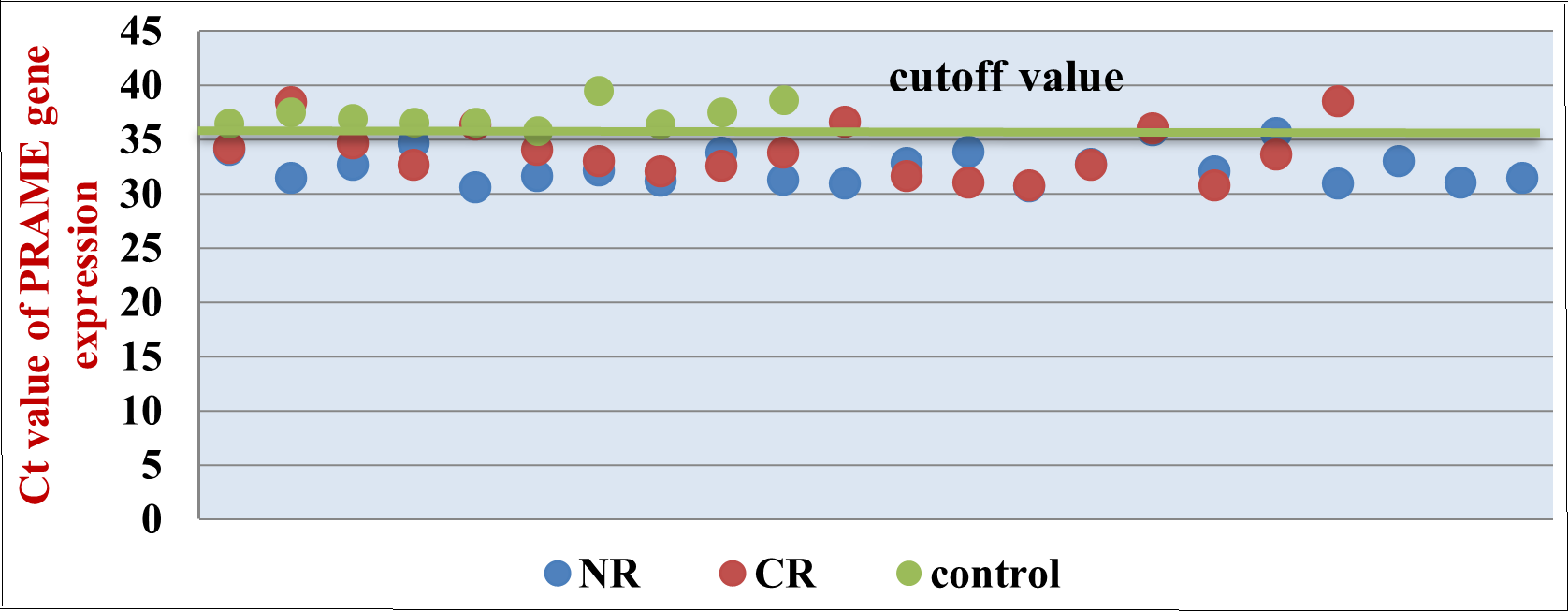
Background: Although PRAME's expression in normal tissue is inconsequential, it is an oncogene in many solid tumors and hematological malignancies; nonetheless, its function and mode of action in acute leukemic cells are still up for discussion. We aimed to expose the relationship between PRAME gene expression and acute leukemia patients with clinical outcomes. Objectives: To examine acute leukemia's expression of the primarily expressed antigen of melanoma "PRAME" and its clinical implications. Methods: A real-time PCR using the Cyber Green test was used to determine the amount of PRAME mRNA expression in peripheral blood cells from 40 patients with acute leukemia and 10 samples from healthy individuals. An analysis of the relationship between the clinical outcome and PRAME gene expression was done. Results: Out of the 50 samples that were obtained, 28% were male and 22% were female. The mean age was 34.3 and 34.3 years for the patients and controls, respectively. Of these, 26(52%) had AML, 14(28%) had ALL, and 10(20%) had voluntary health control. In AL patients, PRAME gene expression was significantly higher (0.643) than in healthy people (0.0468). There were no significant differences between the various types of AL. However, the PRAME mRNA levels showed statistically significant correlation with clinical outcomes. Conclusions: In AML, the PRAME gene is highly expressed, and it may be a helpful indicator for monitoring minimal residual illness; on the other hand, it was linked to a poor prognosis for AML patients.
Downloads
References
De KI, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016; 6(7):e441–e441. doi: 10.1038/bcj.2016.50. DOI: https://doi.org/10.1038/bcj.2016.50
Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. doi: 10.1136/bmj.n2026. DOI: https://doi.org/10.1136/bmj.n2026
Yang J, Chen M, Ye J, Ma H. Targeting PRAME for acute myeloid leukemia therapy. Front Immunol. 2024;15:1378277. doi: 10.3389/fimmu.2024.1378277. DOI: https://doi.org/10.3389/fimmu.2024.1378277
Da Silveira HG, Junior HF, de Souza Campos LD, Stall1 J, Blasius R, Kricheski C, et al. PRAME immunohistochemistry distinguishes nodal nevi from metastatic melanoma. Surg Exp Pathol. 2024;7:28. doi: 10.1186/s42047-024-00171-3. DOI: https://doi.org/10.1186/s42047-024-00171-3
Ikeda H, Lethé B, Lehmann F, van Baren N, Baurain JF, de Smet C, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6(2):199-208. doi: 10.1016/s1074-7613(00)80426-4. DOI: https://doi.org/10.1016/S1074-7613(00)80426-4
Wadelin F, Fulton J, McEwan PA, Spriggs KA, Emsley J, Heery DM. Leucine-rich repeat protein PRAME: expression, potential functions and clinical implications for leukaemia. Mol Cancer. 2010;9:226. doi: 10.1186/1476-4598-9-226. DOI: https://doi.org/10.1186/1476-4598-9-226
Lezcano C, Jungbluth AA, Busam KJ. Comparison of Immunohistochemistry for PRAME With Cytogenetic Test Results in the Evaluation of Challenging Melanocytic Tumors. Am J Surg Pathol. 2020;44(7):893-900. doi: 10.1097/PAS.0000000000001492. DOI: https://doi.org/10.1097/PAS.0000000000001492
Xu Y, Zou R, Wang J, Wang ZW, Zhu X. The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer. Cell Prolif. 2020;53(3):e12770. doi: 10.1111/cpr.12770. DOI: https://doi.org/10.1111/cpr.12770
Güre AO, Stockert E, Arden KC, Boyer AD, Viars CS, Scanlan MJ, et al. CT10: A new cancer-testis (CT) antigen homologous to CT7 and the MAGE family, identified by representational-difference analysis. Int J Cancer. 2000;85(5):726-732. doi: 10.1002/(sici)1097-0215(20000301)85:5<726::aid-ijc21>3.0.co;2-f. DOI: https://doi.org/10.1002/(SICI)1097-0215(20000301)85:5<726::AID-IJC21>3.0.CO;2-F
Paydas S, Tanriverdi K, Yavuz S, Disel U, Baslamisli F, Burgut R. PRAME mRNA levels in cases with acute leukemia: clinical importance and future prospects. Am J Hematol. 2005;79(4):257-261. doi: 10.1002/ajh.20425. DOI: https://doi.org/10.1002/ajh.20425
Kulkarni NV, Shetty RA, Kumari NS, Shetty VV, Krishna R, et al. Correlation of preferentially expressed antigen of melanoma (PRAME) gene expression with clinical characteristics in acute leukemia Patients. J Genet Engineer Biotechnol. 2022;20:97. doi: 10.1186/s43141-022-00376-7. DOI: https://doi.org/10.1186/s43141-022-00376-7
Tanaka N, Wang YH, Shiseki M, Takanashi M, Motoji T. Inhibition of PRAME expression causes cell cycle arrest and apoptosis in leukemic cells. Leuk Res. 2011;35:1219–1225. doi: 10.1016/j.leukres.2011.04.005. DOI: https://doi.org/10.1016/j.leukres.2011.04.005
Oehler VG, Guthrie KA, Cummings CL, Sabo K, Wood BL, Gooley T, et al. The preferentially expressed antigen in melanoma (PRAME) inhibits myeloid differentiation in normal hematopoietic and leukemic progenitor cells. Blood. 2009;114:3299–3308. doi: 10.1182/blood-2008-07-170282. DOI: https://doi.org/10.1182/blood-2008-07-170282
Grillini M, Ricci C, Pino V, Pedrini S, Fiorentino M, Corti B. HMB45/PRAME, a novel double staining for the diagnosis of melanocytic neoplasms: Technical aspects, results, and comparison with other commercially available staining (PRAME and Melan A/PRAME). Appl Immunohistochem Mol Morphol. 2022;30(1):14-18. doi: 10.1097/PAI.0000000000000972. DOI: https://doi.org/10.1097/PAI.0000000000000972
Ricci C, Altavilla MV, Corti B, Pasquini E, Presutti L, Baietti AM, et al. PRAME expression in mucosal melanoma of the head and neck region. Am J Surg Pathol. 2023;47(5):599-610. doi: 10.1097/PAS.0000000000002032. DOI: https://doi.org/10.1097/PAS.0000000000002032
Innocenti L, Scarpitta R, Corraro S, Ortenzi V, Bonadio AG, Loggini B, et al. Shedding light on PRAME expression in dysplastic nevi: a cohort study. Virchows Arch. 2024;485(1):97-104. doi: 10.1007/s00428-023-03720-5. DOI: https://doi.org/10.1007/s00428-023-03720-5
Lezcano C, Jungbluth AA, Busam KJ. PRAME immunohistochemistry as an ancillary test for the assessment of melanocytic lesions. Surg Pathol Clin. 2021;14(2):165–175. doi: 10.1016/j.path.2021.01.001. DOI: https://doi.org/10.1016/j.path.2021.01.001
Cazzato G, Mangialardi K, Falcicchio G, Colagrande A, Ingravallo G, Arezzo F, et al. Preferentially expressed antigen in melanoma (PRAME) and human malignant melanoma: A retrospective study. Genes (Basel). 2022;13(3):545. doi: 10.3390/genes13030545. DOI: https://doi.org/10.3390/genes13030545
Cassalia F, Danese A, Tudurachi I, Federico S, Zambello A, Guidotti A, et al. PRAME updated: Diagnostic, prognostic, and therapeutic role in skin cancer. Int J Mol Sci. 2024;25(3):1582. doi: 10.3390/ijms25031582. DOI: https://doi.org/10.3390/ijms25031582
Proto-Siqueira R, Falco RP, de Souza CA, Jose Ismael S, Zago MA. The expression of PRAME in chronic lymphoproliferative disorders. Leuk Res. 2003; 27:393–396. doi: 10.1016/s0145-2126(02)00217-5. DOI: https://doi.org/10.1016/S0145-2126(02)00217-5
Matsushita M, Ikeda H, Kizaki M, Okamoto S, Ogasawara M, Ikeda Y, et al. Quantitative monitoring of the PRAME gene for the detection of minimal residual disease in leukaemia. Br J Haematol. 2001;112(4):916-926. doi: 10.1046/j.1365-2141.2001.02670.x. DOI: https://doi.org/10.1046/j.1365-2141.2001.02670.x
Huh HJ, Park CJ, Jang S, Seo EJ, Chi HS, Lee JH, et al. Prognostic significance of multidrug resistance gene 1 (MDR1), multidrug resistance-related protein (MRP) and lung resistance protein (LRP) mRNA expression in acute leukemia. J Korean Med Sci. 2006;21(2):253-258. doi: 10.3346/jkms.2006.21.2.253. DOI: https://doi.org/10.3346/jkms.2006.21.2.253
Livak KJ. Relative quantification of gene expression: ABI Prism 7700 sequence detection system. Appl Biosys User Bull. 1997; 2.(updated 2001).
Epping MT, Wang L, Plumb JA, Lieb M, Gronemeyer H, Brown R, et al. A functional genetic screen identifies retinoic acid signaling as a target of histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2007;104(45):17777-17782. doi: 10.1073/pnas.0702518104. DOI: https://doi.org/10.1073/pnas.0702518104
Kewitz S, Staege MS. Knock-down of PRAME increases retinoic acid signaling and cytotoxic drug sensitivity of Hodgkin lymphoma cells. PLoS One. 2013;11;8(2):e55897. doi: 10.1371/journal.pone.0055897. DOI: https://doi.org/10.1371/journal.pone.0055897
Kern CH, Yang M, Liu WS. The PRAME family of cancer testis antigens is essential for germline development and gametogenesis. Biol Reprod. 2021;3;105(2):290-304. doi: 10.1093/biolre/ioab074. DOI: https://doi.org/10.1093/biolre/ioab074
Cakir Y, Lebe B. The relationship of PRAME expression with clinicopathologic parameters and immunologic markers in melanomas: In silico analysis. Appl Immunohistochem Mol Morphol. 2025. doi:10.1097/PAI.0000000000001242. DOI: https://doi.org/10.1097/PAI.0000000000001242
Ding K, Wang XM, Fu R, Ruan E-b, Liu H, Shao ZH. PRAME gene expression in acute leukemia and its clinical significance. Cancer Biol Med. 2012;9(1):73-76. doi: 10.3969/j. issn.2095-3941.2012. 01. 013.
Ortmann CA, Eisele L, Nückel H, Klein-Hitpass L, Führer A, Dührsen U, et al. Aberrant hypomethylation of the cancer-testis antigen PRAME correlates with PRAME expression in acute myeloid leukemia. Ann Hematol. 2008;87(10):809-818. doi: 10.1007/s00277-008-0514-8. DOI: https://doi.org/10.1007/s00277-008-0514-8
Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, Jose-Eneriz ES, et al. Epigenetic regulation of PRAME gene in chronic myeloid leukemia. Leuk Res. 2007;31(11):1521-1528. doi: 10.1016/j.leukres.2007.02.016. DOI: https://doi.org/10.1016/j.leukres.2007.02.016
Santamaría CM, Chillón MC, García-Sanz R, Pérez C, Caballero MD, Ramos F, et al. Molecular stratification model for prognosis in cytogenetically normal acute myeloid leukemia. Blood. 2009;114(1):148-152. doi: 10.1182/blood-2008-11-187724. DOI: https://doi.org/10.1182/blood-2008-11-187724
Santamaría C, Chillón MC, García-Sanz R, Balanzategui A, Sarasquete ME, Alcoceba M, et al. The relevance of preferentially expressed antigen of melanoma (PRAME) as a marker of disease activity and prognosis in acute promyelocytic leukemia. Haematologica. 2008;93(12):1797-1805. doi: 10.3324/haematol.13214. DOI: https://doi.org/10.3324/haematol.13214
Zhang YH, Lu AD, Yang L, Li LD, Chen WM, Long LY, et al. PRAME overexpression predicted good outcome in pediatric B-cell acute lymphoblastic leukemia patients receiving chemotherapy. Leuk Res. 2017;52:43-49. doi: 10.1016/j.leukres.2016.11.005. DOI: https://doi.org/10.1016/j.leukres.2016.11.005
Xu Y, Rong LJ, Meng SL, Hou FL, Zhang JH, Pan G. PRAME promotes in vitro leukemia cells death by regulating S100A4/p53 signaling. Eur Rev Med Pharmacol Sci. 2016;20(6):1057-1063. PMID: 27049257.
Xu Y, Yue Q, Wei H, Pan G. PRAME induces apoptosis and inhibits proliferation of leukemic cells in vitro and in vivo. Int J Clin Exp Pathol. 2015;8(11):14549-14555. PMID: 26823776.
Alyaqubi KJ, Dosh RH, Al-Fatlawi RB, Al-Rehemi SM, Hadi NR. Gene expression of carbonic anhydrase 9 (CA9) in de novo acute leukemia as a predictive marker for prognosis. J Med Life. 2022;15(9):1158-1163. doi: 10.25122/jml-2021-0212. DOI: https://doi.org/10.25122/jml-2021-0212
Alyaqubi KJ, Al-Bedariy IH, Sharba ZF, Jwad Taher TM, Taher MF, Ahmed HA. Wilms’ tumor 1 gene expression as a predictive marker for clinical outcome of acute myeloid leukemia in Iraqi population: A prospective study. Al-Rafidain J Med Sci. 2024;7(2):127-132. doi: 10.54133/ajms.v7i2.1410. DOI: https://doi.org/10.54133/ajms.v7i2.1410
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











