Analysis of Follicular Fluid Fatty Acids in Iraqi Women Undergoing Intracytoplasmic Sperm Injection
DOI:
https://doi.org/10.54133/ajms.v7i1.1116Keywords:
Embryo quality, Fatty acids, Fertilization rate, Oocyte maturation rateAbstract
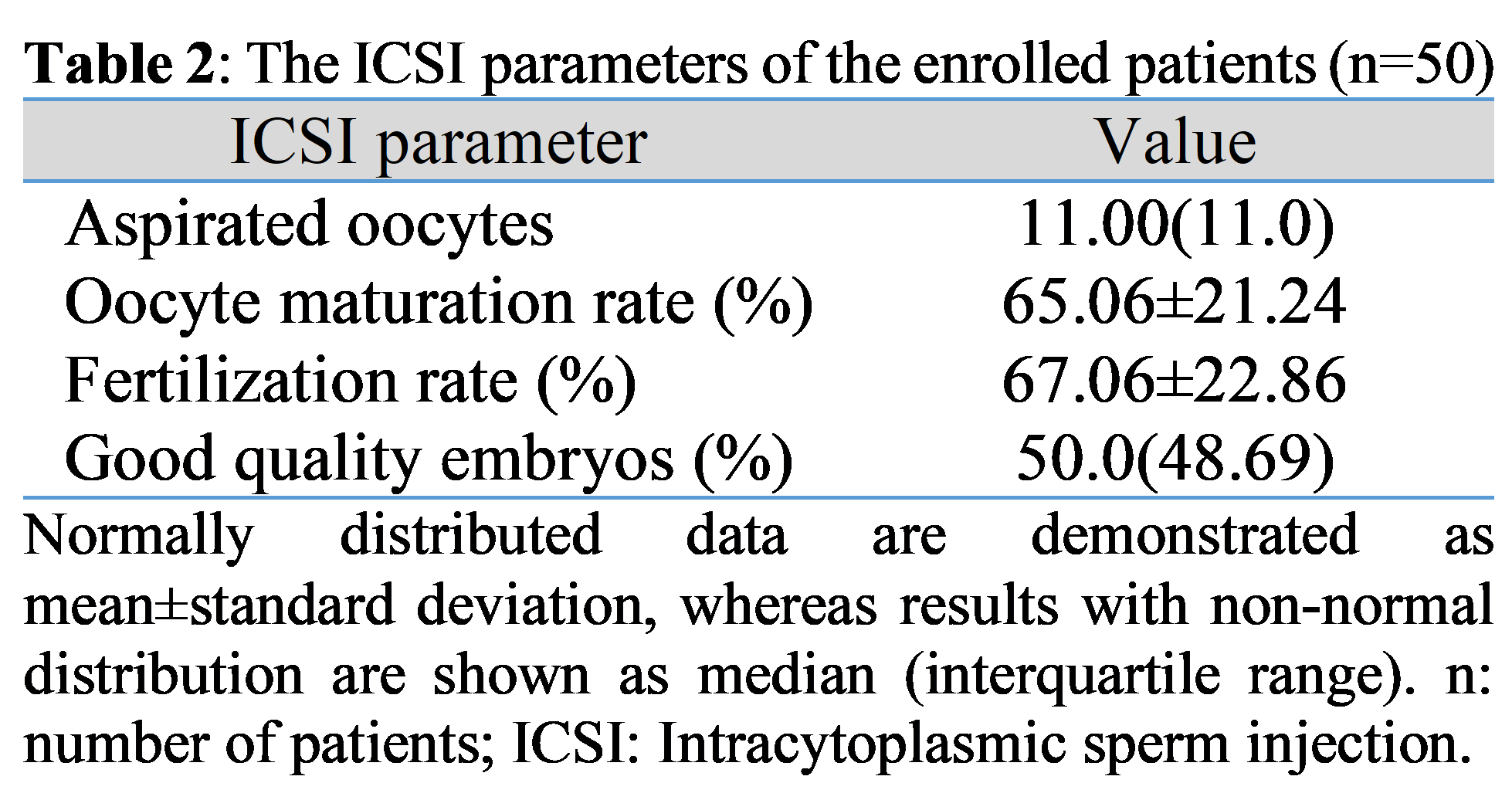
Background: Oocytes are susceptible to alterations in the various fatty acid contents of follicular fluid (FF), which may influence maturation and embryogenesis. Different fatty acids exert various effects on intracytoplasmic sperm injection (ICSI), which needs further studies to uncover the involved mechanisms. Objectives: To assess FF fatty acids in women undergoing ICSI and to correlate them with ICSI parameters, namely the total count of aspirated oocytes, oocyte maturation rate, fertilization rate and percentage of good-quality embryos. Methods: Fifty women undergoing ICSI were enrolled in this cross-sectional study. FF samples were collected during oocyte retrieval and were analyzed for fatty acids using gas chromatography. Fatty acids were calculated as percentages of the total fatty acids. Results: The most common fatty acids found in the FF of women who underwent ICSI were palmitic acid, stearic acid, and oleic acid, with median (interquartile range) of 58.61%(21.66%), 26.27%(14.31%), and 20.13%(31.05%), respectively. Palmitic acid correlated inversely and significantly with oocyte maturation rate, fertilization rate, and percentage of good-quality embryos, with p=0.003, 0.037, and 0.028, respectively. Stearic acid correlated negatively and significantly with oocyte maturation rate (p=0.037) and fertilization rate (p=0.041). Furthermore, an inversely significant correlation was noticed between propionic acid and the percentage of good-quality embryos, as indicated by p=0.014. Conclusions: Palmitic, stearic, and propionic acids in the FF might influence ICSI parameters; thus, they might be used as markers of oocyte developmental competence. Nevertheless, further research is warranted.
Downloads
References
Alrawi QAT, Al-Issa YAH. The Effect of recombinant FSH treatment on ceruloplasmin activity in infertility women undergoing IVF/ICSI. J Pharm Negat Results. 2022;13(special issue 08):1392-1398. doi: 10.47750/pnr.2022.13.S08.171.
Abdul-Kader RA, Ajeel NA. Female infertility: A study of risk factors. J Fac Med Baghdad. 2014;56(2):200-204. doi: 10.32007/jfacmedbagdad.562469. DOI: https://doi.org/10.32007/jfacmedbagdad.562469
Hanon MS, Mazhir SN, Al-Ahmed HI, Haddad RA. Influence of non-thermal plasma (DBD) on infertility male semen with low sperm motility and DNA damage. Iraqi J Sci. 2022;63(4):1491-1497. doi: 10.24996/ijs.2022.63.4.9. DOI: https://doi.org/10.24996/ijs.2022.63.4.9
Salman FS, Al-Qadhi HI, Al Kareem BA. N-acetyl cysteine’s effect on semen parameters in a sample of Iraqi men with oligoasthenoteratozoospermia. J Fac Med Baghdad. 2022;64(3):170-174. doi: 10.32007/jfacmedbagdad.6431938. DOI: https://doi.org/10.32007/jfacmedbagdad.6431938
Shalal MM, Miran NM, Mahddi ZI. Serum leptin levels in ovarian polycystic disease and its correlation to body weight. J Fac Med Baghdad. 2014;56(2):141-146. doi: 10.32007/jfacmedbagdad.562454. DOI: https://doi.org/10.32007/jfacmedbagdad.562454
Ibrahim WW, Kadhim EJ, Abbas NS, Younis SR, Fawzi HA. Serological markers of autoimmunity in women with polycystic ovary syndrome. Int J Res Pharm Sci. 2019;10(3):1746-1750. doi: 10.26452/ijrps.v10i3.1366. DOI: https://doi.org/10.26452/ijrps.v10i3.1366
Hamdi RA, Mohammed NS, AL-Naddawi AM. Determination of serum adiponectin levels in normal weight women with polycystic ovary syndrome. J Fac Med Baghdad. 2015;57(2):175-178. doi: 10.32007/jfacmedbagdad.572352. DOI: https://doi.org/10.32007/jfacmedbagdad.572352
Hassan HH, Ghazi SM, Nasif AS. Study of a hormonal assay in PCOS patients with type 2 DM and their correlation with inhibin B. Medico-Legal Update. 2020;20(3):575-580. doi: 10.37506/mlu.v20i3.1461. DOI: https://doi.org/10.37506/mlu.v20i3.1461
Shallal MM, Meran NM, Hussein ZA. Total L-carnitine and insulin resistance in non-obese and obese Iraqi women with polycystic ovary syndrome. J Fac Med Baghdad. 2023;65(1):20-26. doi: 10.32007/jfacmedbagdad.6512040. DOI: https://doi.org/10.32007/jfacmedbagdad.6512040
Jasim RA, Umran MA, Humadi EH. Correlation between serum interleukins levels with anthropometric data and lipid profiles in obese Iraqi women with polycystic ovary syndrome. Iraqi J Sci. 2020;61(1):68-76. doi: 10.24996/ijs.2020.61.1.7.
Ghalib MM, Rasheed MK, Al-Naddawi AM. Association of neuregulin-4 levels and body mass index with hyperandrogenism in polycystic ovary syndrome patients. J Fac Med Baghdad. 2024;65(4):279-285. doi: 10.32007/jfacmedbagdad.2140. DOI: https://doi.org/10.32007/jfacmedbagdad.2140
Ascar IF, Hameed AS. Serum prolactin, preptin, CCL 18 and genetic polymorphisms in Iraqi women with polycystic ovary syndrome. Baghdad Sci J. 2021;18(4 Suppl.):1552-1556. doi: 10.21123/bsj.2021.18.4(Suppl.).1552. DOI: https://doi.org/10.21123/bsj.2021.18.4(Suppl.).1552
Yin J, Chang HM, Li R, Leung PCK. Recent progress in the treatment of women with diminished ovarian reserve. Gynecol Obstet Clin Med. 2021;1(4):186-189. doi: 10.1016/j.gocm.2021.10.004. DOI: https://doi.org/10.1016/j.gocm.2021.10.004
Zhu Q, Li Y, Ma J, Ma H, Liang X. Potential factors result in diminished ovarian reserve: a comprehensive review. J Ovarian Res. 2023;16(208). doi: 10.1186/s13048-023-01296-x. DOI: https://doi.org/10.1186/s13048-023-01296-x
Kadhum BS, Al-Shammaree SAW. Association of iron status in follicular fluid with pregnancy outcomes in infertile women undergoing IVF/ICSI. Iraqi J Sci. 2021;62(6):1779-1786. doi: 10.24996/ijs.2021.62.6.3. DOI: https://doi.org/10.24996/ijs.2021.62.6.3
Baddela VS, Sharma A, Vanselow J. Non-esterified fatty acids in the ovary: friends or foes?. Reprod Biol Endocrinol. 2020;18(60). doi: 10.1186/s12958-020-00617-9. DOI: https://doi.org/10.1186/s12958-020-00617-9
Hewavitharana GG, Perera DN, Navaratne SB, Wickramasinghe I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab J Chem. 2020;13(8):6865-6875. doi: 10.1016/j.arabjc.2020.06.039. DOI: https://doi.org/10.1016/j.arabjc.2020.06.039
Zeng X, Li S, Liu L, Cai S, Ye Q, Xue B, et al. Role of functional fatty acids in modulation of reproductive potential in livestock. J Anim Sci Biotechnol. 2023;14(24). doi: 10.1186/s40104-022-00818-9. DOI: https://doi.org/10.1186/s40104-022-00818-9
Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, et al. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis. 2017;16(18). doi: 10.1186/s12944-016-0396-z. DOI: https://doi.org/10.1186/s12944-016-0396-z
Zhou X, Mo Z, Li Y, Huang L, Yu S, Ge L, et al. Oleic acid reduces steroidogenesis by changing the lipid type stored in lipid droplets of ovarian granulosa cells. J Anim Sci Biotechnol. 2022;13(27). doi: 10.1186/s40104-021-00660-5. DOI: https://doi.org/10.1186/s40104-021-00660-5
Hanon MS, Mazhir SN, Al-Ahmed HI, Hussein EA. Effect of cold atmospheric pressure plasma on DNA integrity in patients with asthenospermia. J Phys Conf Ser. 2019;1178:012029. doi: 10.1088/1742-6596/1178/1/012029. DOI: https://doi.org/10.1088/1742-6596/1178/1/012029
Al-Hamedawi TM, Zalzala SJ, Al-Shammary SM. Biochemical composition of caprine follicular fluid in relation with different follicles size in Iraqi local goats. Adv Anim Vet Sci. 2017;5(3):145-147. doi: 10.14737/journal.aavs/2017/5.3.145.147. DOI: https://doi.org/10.14737/journal.aavs/2017/5.3.145.147
Al-Omary HL, Alawad ZM, Husseini B. Cell-free DNA as a clinical indicator in maternal blood. Turk J Endocrinol Metab. 2019;23(3):174-180. doi: 10.25179/tjem.2019-65572. DOI: https://doi.org/10.25179/tjem.2019-65572
Ishak GM, Feugang JM, Pechanova O, Pechan T, Peterson DG, Willard ST, et al. Follicular fluid proteomics during equine follicle development. Mol Reprod Dev. 2022;89(7):298-311. doi: 10.1002/mrd.23622. DOI: https://doi.org/10.1002/mrd.23622
The Rotterdam ESHRE/ASRM sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47. doi: 10.1093/humrep/deh098. DOI: https://doi.org/10.1093/humrep/deh098
Fadhil NM, Hamdi RA. Evaluation of serum podocalyxin in Iraqi women with polycystic ovary syndrome. J Fac Med Baghdad. 2023;64(4):277-280. doi: 10.32007/jfacmedbagdad.6441983. DOI: https://doi.org/10.32007/jfacmedbagdad.6441983
Hamdi RA, Abdul-Qahar ZH, Kadhum EJ, Alsaeed FA. Assessment of serum vitamin D levels in women with polycystic ovary syndrome. J Fac Med Baghdad. 2018;60(2):93-97. doi: 10.32007/jfacmedbagdad.60212. DOI: https://doi.org/10.32007/19jfacmedbaghdad36.v60i2.12
Ferraretti AP, La Marca A, Fauser BCJM, Tarlatzis B, Nargund G, Gianaroli L. ESHRE consensus on the definition of 'poor response' to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod. 2011;26(7):1616-1624. doi: 10.1093/humrep/der092. DOI: https://doi.org/10.1093/humrep/der092
Al-Rubae'i SHN, Naji TS, Turki KM, Edan DS. Association of the G/T rs4646 of CYP19 gene polymorphism with oxidative stress, vitamin A and estradiol in Iraqi women with endometriosis disease. Gene Rep. 2018;11:12-17. doi: 10.1016/j.genrep.2018.01.005. DOI: https://doi.org/10.1016/j.genrep.2018.01.005
Hatem A, Saleh BO, Al-Naddawi AM. Association between serum fructose level and insulin resistance in women with polycystic ovary syndrome: The effect of obesity. J Fac Med Baghdad. 2022;64(2):91-95. doi: 10.32007/jfacmedbagdad.6421926. DOI: https://doi.org/10.32007/jfacmedbagdad.6421926
Hasan AE, Taqa LR, Saeed GT. Correlation of body mass index with tissue Doppler parameters in obese middle age subjects. Ann Trop Med Public Health. 2020;23(S18):SP231822. doi: 10.36295/ASRO.2020.231822. DOI: https://doi.org/10.36295/ASRO.2020.231822
Alawad ZM. Level of follicular fluid vitamin D and embryo quality in a sample of Iraqi women undergoing IVF. J Fac Med Baghdad. 2019;60(4):215-221. doi: 10.32007/jfacmedbagdad.604758. DOI: https://doi.org/10.32007/jfacmedbagdad.604758
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270-1283. doi: 10.1093/humrep/der037. DOI: https://doi.org/10.1016/j.rbmo.2011.02.001
Mirabi P, Chaichi MJ, Esmaeilzadeh S, Jorsaraei SGA, Bijani A, Ehsani M. Does different BMI influence oocyte and embryo quality by inducing fatty acid in follicular fluid? Taiwan J Obstet Gynecol. 2017;56(2):159-164. doi: 10.1016/j.tjog.2016.11.005. DOI: https://doi.org/10.1016/j.tjog.2016.11.005
Kermack AJ, Wellstead SJ, Fisk HL, Cheong Y, Houghton FD, Macklon NS, et al. The fatty acid composition of human follicular fluid is altered by a 6 week dietary intervention that includes marine omega-3 fatty acids. Lipids. 2021;56(2):201-209. doi: 10.1002/lipd.12288. DOI: https://doi.org/10.1002/lipd.12288
Shaaker M, Rahimipour A, Nouri M, Khanaki K, Darabi M, Farzadi L, et al. Fatty acid composition of human follicular fluid phospholipids and fertilization rate in assisted reproductive techniques. Iran Biomed J. 2012;16(3):162-168. doi: 10.6091/ibj.1081.2012.
Valckx SD, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, et al. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: a descriptive cross-sectional study. Reprod Biol Endocrinol. 2014;12(13). doi: 10.1186/1477-7827-12-13. DOI: https://doi.org/10.1186/1477-7827-12-13
Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, et al. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS One. 2011;6(8):e23183. doi: 10.1371/journal.pone.0023183. DOI: https://doi.org/10.1371/journal.pone.0023183
Al-Rudaini AT, Al-Dujaily SS, Salih LA. A comparative study of preimplantation embryos development of young and aged mice treated with L-carnitine. Baghdad Sci J. 2023. doi: 10.21123/bsj.2023.8923. DOI: https://doi.org/10.21123/bsj.2023.8923
Chen Z, Lei L, Wen D, Yang L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J Ovarian Res. 2019;12(1):43. doi: 10.1186/s13048-019-0519-z. DOI: https://doi.org/10.1186/s13048-019-0519-z
Jungheim ES, Louden ED, Chi MM-Y, Frolova AI, Riley JK, Moley KH. Preimplantation exposure of mouse embryos to palmitic acid results in fetal growth restriction followed by catch-up growth in the offspring. Biol Reprod. 2011;85(4):678-683. doi: 10.1095/biolreprod.111.092148. DOI: https://doi.org/10.1095/biolreprod.111.092148
Warzych E, Cieslak A, Pawlak P, Renska N, Pers-Kamczyc E, Lechniak D. Maternal nutrition affects the composition of follicular fluid and transcript content in gilt oocytes. Vet Med. 2011;56(4):156-67. doi: 10.17221/1573-VETMED. DOI: https://doi.org/10.17221/1573-VETMED
Liu Y, Tilleman K, Vlaeminck B, Gervais R, Chouinard PY, De Sutter P, et al. The fatty acid composition in follicles is related to the developmental potential of oocytes up to the blastocyst stage: a single-centre cohort study. Reprod Biol Endocrinol. 2022;20(107). doi: 10.1186/s12958-022-00974-7. DOI: https://doi.org/10.1186/s12958-022-00974-7
Fayezi S, Leroy JLMR, Ghaffari Novin M, Darabi M. Oleic acid in the modulation of oocyte and preimplantation embryo development. Zygote. 2018;26(1):1-13. doi: 10.1017/S0967199417000582. DOI: https://doi.org/10.1017/S0967199417000582
Lee JE, Yong H, Kim HY, Lee WH, Cheong HT, Yang BK, et al. Effect of alpha-linolenic acid on oocyte maturation and embryo development in pigs. Dev Reprod. 2017;21(2):205-213. doi: 10.12717/DR.2017.21.2.205. DOI: https://doi.org/10.12717/DR.2017.21.2.205
Amini E, Asadpour R, Roshangar L, Jafari-Joozani R. Effect of linoleic acid supplementation on in vitro maturation, embryo development and apoptotic related gene expression in ovine. Int J Reprod Biomed. 2016;14(4):255-262. PMID: 27351027. DOI: https://doi.org/10.29252/ijrm.14.4.255
Olaniyi KS, Bashir AM, Areloegbe SE, Sabinari IW, Akintayo CO, Oniyide AA, et al. Short chain fatty acid, acetate restores ovarian function in experimentally induced PCOS rat model. PLoS One. 2022;17(7):e0272124. doi: 10.1371/journal.pone.0272124. DOI: https://doi.org/10.1371/journal.pone.0272124
Tirosh A, Calay ES, Tuncman G, Claiborn KC, Inouye KE, Eguchi K, et al. The short-chain fatty acid propionate increases glucagon and FABP4 production, impairing insulin action in mice and humans. Sci Transl Med. 2019;11(489):eaav0120. doi: 10.1126/scitranslmed.aav0120. DOI: https://doi.org/10.1126/scitranslmed.aav0120
Shi M, Sirard MA. Metabolism of fatty acids in follicular cells, oocytes, and blastocysts. Reprod Fertil. 2022;3(2):R96-R108. doi: 10.1530/RAF-21-0123. DOI: https://doi.org/10.1530/RAF-21-0123
Liu Y, Tilleman K, Vlaeminck B, Gervais R, Chouinard PY, De Sutter P, et al. Composition and distribution of fatty acids in various lipid fractions in serum and follicular fluid of women undergoing assisted reproductive technology. PLoS One. 2023;18(6):e0286946. doi: 10.1371/journal.pone.0286946. DOI: https://doi.org/10.1371/journal.pone.0286946
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











