Comparative Role of Heat Shock Protein 70 and tTG Autoantibodies as Biomarkers in Diagnosis and Follow-up of Marsh 3 Celiac Disease
DOI:
https://doi.org/10.54133/ajms.v9i2.2551Keywords:
Anti-tTG, Celiac disease serology, Heat shock protein-70, Marsh classificationAbstract
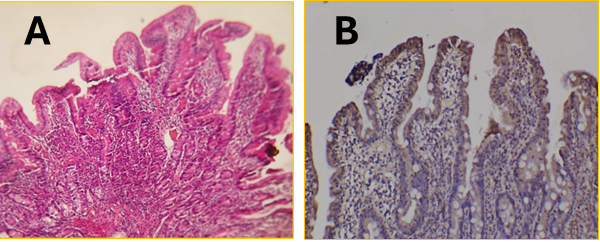
Background: Celiac disease (CD) is an autoimmune disorder characterized by an abnormal immune response to gluten, leading to intestinal mucosal damage. Anti-tissue transglutaminase (anti-tTG) antibodies are well-known ways to diagnose CD. However, it is still not clear what role heat shock protein 70 (HSP70) plays in the disease and protecting the mucosa. Objective: To evaluate serum and tissue levels of anti-tTG antibodies and HSP70 in patients with celiac disease and assess their potential as biomarkers for disease diagnosis and progression. Methods: This case-control study included 64 CD patients and 26 healthy controls from Al-Anbar province. Anti-tTG IgA and IgG serum levels were analyzed, and HSP70 tissue expression was assessed in samples from a duodenal biopsy. Histopathological findings were graded according to the Marsh classification. Results: CD was more prevalent in females (56.5%) than males (37.5%). Serum anti-tTG IgA and IgG levels were significantly elevated in CD patients compared to controls and correlated with the degree of intestinal damage. Both antibodies showed a progressive increase from Marsh 3A to 3C, with Marsh 3C exhibiting the highest levels (tTG-IgA: 19.12 µg/mL; tTG-IgG: 13.44 µg/mL). In contrast, HSP70 tissue expression was low or absent in 53.2% of CD patients, particularly in those with severe mucosal injury (Marsh 3C). Conclusions: Anti-tTG antibodies remain useful for confirming CD diagnosis but are limited in predicting disease progression. HSP70 expression, which declines with increasing mucosal damage, may serve as a complementary biomarker reflecting cellular stress, mucosal integrity, and early disease activity.
Downloads
References
Herrera-Quintana L, Navajas-Porras B, Vázquez-Lorente H, Hinojosa-Nogueira D, Corrales-Borrego FJ, Lopez-Garzon M, et al. Celiac Disease: Beyond diet and food awareness. Foods. 2025;14(3):377. doi: 10.3390/foods14030377. DOI: https://doi.org/10.3390/foods14030377
King JA, Jeong J, Underwood FE, Quan J, Panaccione N, Windsor JW, et al. Incidence of celiac disease is increasing over time: A systematic review and meta-analysis. Am J Gastroenterol. 2020;115(4):507-525. doi: 10.14309/ajg.0000000000000523. DOI: https://doi.org/10.14309/ajg.0000000000000523
Catassi GN, Pulvirenti A, Monachesi C, Catassi C, Lionetti E. Diagnostic accuracy of IgA anti-transglutaminase and IgG anti-deamidated gliadin for diagnosis of celiac disease in children under two years of age: A systematic review and meta-analysis. Nutrients. 2022;14(1):7. doi: 10.3390/nu14010007. DOI: https://doi.org/10.3390/nu14010007
Volta U, Bai JC, De Giorgio R. The role of serology in the diagnosis of coeliac disease. Gastroenterol Hepatol Bed Bench. 2023;16(2):118. doi: 10.22037/ghfbb.v16i2.2713.
Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, et al. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm. 2022;3:e161. doi: 10.1002/mco2.161. DOI: https://doi.org/10.1002/mco2.161
De Oliveira AA, Mendoza VO, Rastogi S, Nunes KP. New insights into the role and therapeutic potential of HSP70 in diabetes. Pharmacol Res. 2022;178:106173. doi: 10.1016/j.phrs.2022.106173. DOI: https://doi.org/10.1016/j.phrs.2022.106173
De Oliveira AA, Priviero F, Webb RC, Nunes KP. Increased EHSP70-to-IHSP70 ratio disrupts vascular responses to calcium and activates the TLR4-MD2 complex in type 1 Diabetes. Life Sci. 2022; 310:121079. doi: 10.1016/j.lfs.2022.121079. DOI: https://doi.org/10.1016/j.lfs.2022.121079
Abbass Qasim Z, Al-Dahhan HA. Assessment the levels of antibodies (Anti-TTG and Anti-AGA) in the serum of patients with celiac disease. Int J Pharm Bio-Med Sci. 2024;4(7). doi: 10.47191/ijpbms/v4-i7-01. DOI: https://doi.org/10.47191/ijpbms/v4-i7-01
Taneja K, Mahajan N, Rai A, Malik S, Khatri A. Association of anti-tissue transglutaminase antibody titers and duodenal biopsy findings in pediatric patients of celiac disease. Cureus. 2021;13(3):e13679. doi: 10.7759/cureus.13679. DOI: https://doi.org/10.7759/cureus.13679
Sedaqat M, Iranikhah A, Aghili B, Akbari Aleagha MM. Association between level anti-tissue transglutaminase antibody titers and duodenal histopathology among patients with celiac disease. Afghan J Basic Med Sci. 2025;2(2):222-228. doi: 10.62134/khatamuni.74. DOI: https://doi.org/10.62134/khatamuni.74
Pollard KM, Cauvi DM, Mayeux JM, Toomey CB, Peiss AK, Hultman P, et al. Mechanisms of environment-induced autoimmunity. Annu Rev Pharmacol Toxicol. 2021;61:135-157. doi: 10.1146/annurev-pharmtox-031320-111453. DOI: https://doi.org/10.1146/annurev-pharmtox-031320-111453
Fontana G, Ziberna F, Barbi E, Di Leo G, De Leo L. Intestinal celiac disease - related autoantibodies. Front Immunol. 2025;16:1567416. doi: 10.3389/fimmu.2025.1567416. DOI: https://doi.org/10.3389/fimmu.2025.1567416
Kowalski MK, Domżał-Magrowska D, Małecka-Wojciesko E. Evaluation of the frequency of HLA-DQ2/DQ8 genes among patients with celiac disease and those on a gluten-free diet. Foods. 2025;14(2):298. doi: 10.3390/foods14020298. DOI: https://doi.org/10.3390/foods14020298
Iversen R, Sollid LM. The immunobiology and pathogenesis of celiac disease. Annu Rev Pathol Mech Dis. 2023;18:47-70. doi: 10.1146/annurev-pathol-052020-121908. DOI: https://doi.org/10.1146/annurev-pathmechdis-031521-032634
Yong Y, Li J, Yu T, Fang B, Liu X, Yu Z, et al. Overexpression of heat shock protein 70 induces apoptosis of intestinal epithelial cells in heat-stressed pigs: A proteomics approach. J Therm Biol. 2022;108:103289. doi: 10.1016/j.jtherbio.2022.103289.. DOI: https://doi.org/10.1016/j.jtherbio.2022.103289
Chen S, Qin R, Fan X, Zhang Z, Zhou L, Wang H. HSP70 protects against acute pancreatitis-elicited intestinal barrier damage in rats. Clin Res Hepatol Gastroenterol. 2024;48(7):102388. doi: 10.1016/j.clinre.2024.102388. DOI: https://doi.org/10.1016/j.clinre.2024.102388
Singh MK, Han S, Ju S, Ranbhise JS, Ha J, Yeo SG, et al. Hsp70: A multifunctional chaperone in maintaining proteostasis and its implications in human disease. Cells. 2025;14(7):509. doi: 10.3390/cells14070509. DOI: https://doi.org/10.3390/cells14070509
Mohtasham N, Shahabinejad M, Kafiroudi S, Mohajertehran F. Evaluation of the altered tissue expression of HSP60 and HSP70 genes in oral and cutaneous lichen planus compared to normal healthy tissues. Indian J Dermatol. 2021;66(6):591-597. doi: 10.4103/ijd.ijd_1060_20. DOI: https://doi.org/10.4103/ijd.ijd_1060_20
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











