Natalizumab Immunogenicity in Multiple Sclerosis: Evaluating Antibody Development and Clinical Outcomes in an Iraqi Cohort
DOI:
https://doi.org/10.54133/ajms.v9i2.2352Keywords:
Anti-natalizumab antibodies, Disease activity, EDSS, Immunogenicity, Multiple sclerosis, NatalizumabAbstract
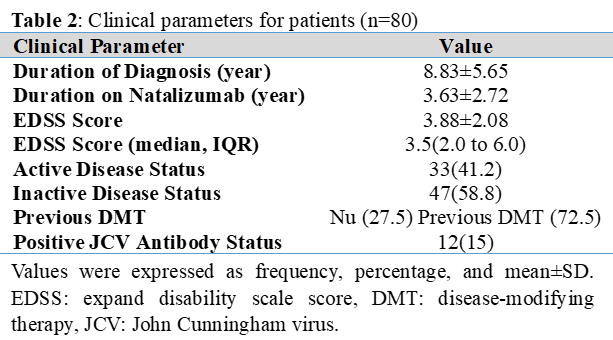
Background: Natalizumab is an effective disease-modifying drug for multiple sclerosis (MS); nevertheless, the formation of anti-natalizumab antibodies (ANA) may reduce therapeutic effectiveness. Objective: To determine the prevalence of ANA, as well as the association between ANA and disease activity and clinical outcome. Methods: This cross-sectional research included 80 MS patients. Demographics and clinical profile, anti-natalizumab antibodies, and JCV status were all evaluated. Disease activity was determined using the Expanded Disability Status Scale (EDSS) and active/inactive disease categorization. To identify predictors of ANA development, correlation and multivariable logistic regression analyses were performed. Results: ANA were detected in 25 individuals (31.25%, 95% CI: 21.4–42.3%). 86% of participants showed ANA during the first 18 months of therapy, with a median time of 14.5 months (IQR: 8.2-22.1 months). Active disease was reported in 33(41.2%) of the 80 participants. There was no significant relationship between ANA levels and disease activity (p=0.927). The mean EDSS scores didn't differ between groups (ANA-positive: 3.70 vs. ANA-negative: 3.96; p=0.576). The ANA-positive cohorts showed reduced EDSS progression (1.58) compared to those with ANA-negative (2.03), although this difference was not statistically significant (p=0.517). Conclusions: Anti-natalizumab antibodies were prevalent in MS patients, although their existence had no meaningful correlation with clinical outcome. There was no link between disease activity and antibody production.
Downloads
References
Rajabi M, Shafaeibajestan S, Asadpour S, Alyari G, Taei N, Kohkalani M, et al. Primary progressive multiple sclerosis: New therapeutic approaches. Neuropsychopharmacol Rep. 2025;45(3):e70039. doi: 10.1002/npr2.70039. DOI: https://doi.org/10.1002/npr2.70039
Wang LY, Wang WF, Hui SY, Yang L, Liu YX, Li HJ. Emerging epidemiological trends of multiple sclerosis among adults aged 20-54 years, 1990-2021, with projections to 2035: a systematic analysis for the global burden of disease study 2021. Front Neurol. 2025;16:1616245. doi: 10.3389/fneur.2025.1616245. DOI: https://doi.org/10.3389/fneur.2025.1616245
Hassoun HK, Al-Mahadawi A, Sheaheed NM, Sami SM, Jamal A, Allebban Z. Epidemiology of multiple sclerosis in Iraq: retrospective review of 4355 cases and literature review. Neurol Res. 2022;44(1):14-23. doi: 10.1080/01616412.2021.1952511. DOI: https://doi.org/10.1080/01616412.2021.1952511
Chaves B, Santos e Silva JC, Nakaya H, Socquet-Juglard N, Bucciarelli F, Prunier G, et al. In vitro morphological profiling of T cells predicts clinical response to natalizumab therapy in patients with multiple sclerosis. Nat Commun. 2025;16(1):5533. doi: 10.1038/s41467-025-60224-3. DOI: https://doi.org/10.1038/s41467-025-60224-3
Meneses AC, da Cunha FPPF, Ribeiro FCP, de Deus Borges L, da Silva Koch SB, Lopes WAD, et al. Disease progression in pregnant women with relapsing–remitting multiple sclerosis treated with fingolimod or natalizumab prior to conception: A systematic review. SN Compr Clin Med. 2025;7(1):1-10. doi: 10.1007/s42399-025-01878-4. DOI: https://doi.org/10.1007/s42399-025-01878-4
Chow HH, Petersen ER, Olsson A, Laursen JH, Hansen MB, Oturai AB, et al. Age-corrected neurofilament light chain ratio decreases but does not predict relapse in highly active multiple sclerosis patients initiating natalizumab treatment. Mult Scler Relat Disord. 2024;88:105701. doi: 10.1016/j.msard.2024.105701. DOI: https://doi.org/10.1016/j.msard.2024.105701
Chamberlain P, Hemmer B, Höfler J, Wessels H, von Richter O, Hornuss C, et al. Comparative immunogenicity assessment of biosimilar natalizumab to its reference medicine: a matching immunogenicity profile. Front Immunol. 2024;15:1414304. doi: 10.3389/fimmu.2024.1414304. DOI: https://doi.org/10.3389/fimmu.2024.1414304
Yang G, Massumi M. Fragment-based immune cell engager antibodies in treatment of cancer, infectious and autoimmune diseases: Lessons and insights from clinical and translational studies. Antibodies. 2025;14(3):52. doi: 10.3390/antib14030052. DOI: https://doi.org/10.3390/antib14030052
Mrochen A, Meuth SG, Pfeuffer S. Should we stay or should we go? Recent insights on drug discontinuation in multiple sclerosis. Neurol Res Pract. 2025;7(1):25. doi: 10.1186/s42466-025-00379-y. DOI: https://doi.org/10.1186/s42466-025-00379-y
Høgestøl EA, Brustad ÅW, Celius EG, Meling M, Berg-Hansen P, Kro GB, et al. Real-world experience with switching from originator to biosimilar natalizumab. medRxiv. 2025:2025-02. doi: 10.1101/2025.02.05.25320428. DOI: https://doi.org/10.1101/2025.02.05.25320428
Gao M, Saito R, Watanabe H, Shimojo T, Akahoshi T, Kuramoto S, et al. Impacts of anti-drug antibodies on pharmacokinetic and pharmacodynamic actions of cell-permeable middle molecule peptide drug. J Pharmacol Exp Ther. 2025;18(1):103663. doi: 10.1016/j.jpet.2025.103663. DOI: https://doi.org/10.1016/j.jpet.2025.103663
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2. DOI: https://doi.org/10.1016/S1474-4422(17)30470-2
Keating PE, Duncan R, Spellerberg M, O'Donnell J, Hock BD. Measurement of anti-natalizumab antibodies by homogeneous mobility shift assay. Pathology. 2020;52(3):373-374. doi: 10.1016/j.pathol.2020.01.682. DOI: https://doi.org/10.1016/j.pathol.2020.01.682
Sørensen PS, Hyldgaard Jensen PE, Haghikia A, Lundkvist M, Vedeler C, Sellebjerg F, et al. Occurrence of antibodies against natalizumab in relapsing multiple sclerosis patients treated with natalizumab. Mult Scler J. 2011;17(9):1074-1078. doi: 10.1177/135245851140427. DOI: https://doi.org/10.1177/1352458511404271
Oliver B, Fernández Ó, Órpez T, Alvarenga MP, Pinto-Medel MJ, Guerrero M, et al. Kinetics and incidence of anti-natalizumab antibodies in multiple sclerosis patients on treatment for 18 months. Mult Scler J. 2011;17(3):368-371. doi: 10.1177/1352458510385508. DOI: https://doi.org/10.1177/1352458510385508
Oliver-Martos B, Orpez-Zafra T, Urbaneja P, Maldonado-Sanchez R, Leyva L, Fernández O. Early development of anti-natalizumab antibodies in MS patients. J Neurol. 2013;260(9):2343-7234. doi: 10.1007/s00415-013-6991-2. DOI: https://doi.org/10.1007/s00415-013-6991-2
Maroufi H, Mortazavi SH, Sahraian MA, Eskandarieh S. Environmental risk factors of multiple sclerosis in the Middle East and North Africa region: a systematic review. Curr J Neurol. 2021;20(3):166. doi: 10.18502/cjn.v20i3.7693. DOI: https://doi.org/10.18502/cjn.v20i3.7693
Ciano-Petersen NL, Aliaga-Gaspar P, Hurtado-Guerrero I, Reyes V, Rodriguez-Bada JL, Rodriguez-Traver E, et al. Natalizumab-immunogenicity evaluation in patients with infusion related events or disease exacerbations. Front Immunol. 2023;14:1242508. doi:10.3389/fimmu.2023.1242508. DOI: https://doi.org/10.3389/fimmu.2023.1242508
Ahmed FT, Ali SH, Al Gawwam GA. Integrin alpha-4 gene polymorphism in relation to natalizumab response in multiple sclerosis patients. Neurol Asia. 2023;28(2). doi: 10.54029/2023afn. DOI: https://doi.org/10.54029/2023afn
Ad'hiah AH, Atiyah NS, Fadhil HY. Qualitative and quantitative molecular analysis of Epstein-Barr virus in Iraqi patients with relapsing-remitting multiple sclerosis. Iraqi J Sci. 2023:127-37. doi: 10.24996/ijs.2023.64.1.13. DOI: https://doi.org/10.24996/ijs.2023.64.1.13
Yawuz MJ, Ahmed MQF, Mohammed SA. Determination of level of 25-hydroxy vitamin D3 in patients with multiple sclerosis and its effect on disease activity. J Pure Appl Microbiol. 2019;13(1):545-551. doi: 10.22207/JPAM.13.1.61. DOI: https://doi.org/10.22207/JPAM.13.1.61
Khoy K, Mariotte D, Defer G, Petit G, Toutirais O, Le Mauff B. Natalizumab in multiple sclerosis treatment: from biological effects to immune monitoring. Front Immunol. 2020;11:549842. doi: 10.3389/fimmu.2020.549842. DOI: https://doi.org/10.3389/fimmu.2020.549842
Subramanyam M. Case study: immunogenicity of natalizumab. Immunogen Biopharm. 2008; 173-87. doi: 10.1007/978-0-387-75841-1_10. DOI: https://doi.org/10.1007/978-0-387-75841-1_10
Vennegoor A, Rispens T, Strijbis EMM, Seewann A, Uitdehaag BMJ, Balk LJ, et al. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler J. 2013;19(5):593-600. doi: 10.1177/135245851246. DOI: https://doi.org/10.1177/1352458512460604
Gorovits B. Current considerations for immunoglobulin isotype characterization of antibody response against biotherapeutics. AAPS J. 2020;22(6):144. doi: 10.1208/s12248-020-00530-4. DOI: https://doi.org/10.1208/s12248-020-00530-4
Cassotta A, Mikol V, Bertrand T, Pouzieux S, Le Parc J, Ferrari P, et al. A single T cell epitope drives the neutralizing anti-drug antibody response to natalizumab in multiple sclerosis patients. Nat Med. 2019;25(9):1402-1407. doi: 10.1038/s41591-019-0568-2. DOI: https://doi.org/10.1038/s41591-019-0568-2
Lundkvist M, Engdahl E, Holmén C, Movérare R, Olsson T, Hillert J, et al. Characterization of anti-natalizumab antibodies in multiple sclerosis patients. Mult Scler J. 2013;19(6):757-764. doi: 10.1177/1352458512462920. DOI: https://doi.org/10.1177/1352458512462920
Lories R. SP0079 Pathologies across the tissues in psa. Ann Rheum Dis. 2017;76:19-20. doi: 10.1136/annrheumdis-2017-eular.7122. DOI: https://doi.org/10.1136/annrheumdis-2017-eular.7258
Jwad RK, Kadhim DJ, Alosami MHM, Shareef LG. Medication-related burden among Iraqi patients with rheumatoid arthritis: An observational study. F1000Research. 2022;11(1047):1047. doi: 10.12688/f1000research.125446.1. DOI: https://doi.org/10.12688/f1000research.125446.1
Ziemssen T, Gass A, Wuerfel J, Bayas A, Tackenberg B, Limmroth V, et al. Design of TRUST, a non-interventional, multicenter, 3-year prospective study investigating an integrated patient management approach in patients with relapsing-remitting multiple sclerosis treated with natalizumab. BMC Neurol. 2016;16(1):98. doi: 10.1186/s12883-016-0625-0. DOI: https://doi.org/10.1186/s12883-016-0625-0
Abna Z. Patient monitoring during treatment with natalizumab (ORP-24). Neurol Lett. 2023;2(Suppl. 1). 20th Iranian Multiple Sclerosis Congress:e184229.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











