Cytotoxicity of Sericin Nanoparticles Loaded with Paclitaxel as a Pulmonary Drug Delivery System: In vitro and in vivo Studies
DOI:
https://doi.org/10.54133/ajms.v7i1.1153Keywords:
Lung dose calculation, Paclitaxel, Pulmonary drug delivery, Self-assembled nanoparticlesAbstract
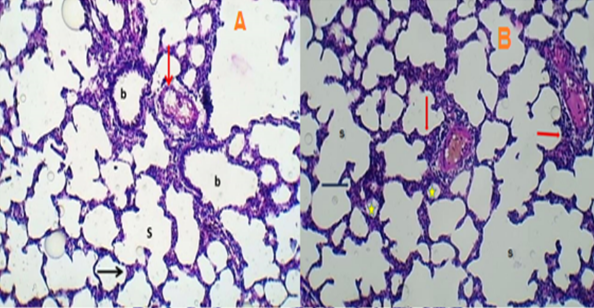
Background: The remarkably low delivery efficiency and lack of specificity of anticancer medicines constrain systemic chemotherapy due to its inadequate therapeutic effectiveness and significant toxic side effects. Objective: To evaluate the feasibility of protein nanoparticles made from sericin and loaded with paclitaxel as a carrier for pulmonary delivery for lung cancer treatment. Methods: Self-assembled nanoparticles made from sericin and poloxamer 407 and loaded with paclitaxel were prepared by the desolvation method and the physicochemical, in vitro and in vivo characteristics of the prepared nanoparticles were investigated. Results: The PTX-loaded sericin nanoparticles were successfully prepared and exhibited low particle size (145.0 nm), high entrapment efficiency of paclitaxel, and spherical shape confirmed by TEM. The nanoparticles demonstrated prolonged cytotoxicity on A549 cells in comparison to the conventional paclitaxel solution. Once transformed into aerosol form, the nanoparticles significantly extended the duration of paclitaxel in the lungs and slowed down its elimination compared to the standard medication (Taxol®). The animal group treated with these nanoparticles did not exhibit any notable histopathological findings when compared to the control animal group. Conclusions: Aerosolized nanoparticles can improve the delivery of paclitaxel to the lungs, leading to improved effectiveness and a lower frequency of medication administration. They also show promise as a therapeutic method for treating lung cancer.
Downloads
References
Chhikara BS, Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chem Biol Lett. 2023;10(1):451.
Cai H, Wang Y, Qin D, Cui Y, Zhang H. Advanced surgical technologies for lung cancer treatment: Current status and perspectives. Engineer Regen. 2023;4(1):55-67. doi: 10.1016/j.engreg.2022.12.001. DOI: https://doi.org/10.1016/j.engreg.2022.12.001
Hashim AA-J, Rajab NA. Anastrozole loaded nanostructured lipid carriers: Preparation and evaluation. Iraqi J Pharm Sci. 2021;30(2):185-195. doi: 10.31351/vol30iss2pp185-19. DOI: https://doi.org/10.31351/vol30iss2pp185-195
Bhattacharya S. Development of 5-FU loaded poly lactic-co-glycolic acid nanoparticles for treatment of lung cancer. Iraqi J Pharm Sci. 2022;31(1):130-143. doi: 10.31351/vol31iss1pp130-143. DOI: https://doi.org/10.31351/vol31iss1pp130-143
Storti C, Le Noci V, Sommariva M, Tagliabue E, Balsari A, Sfondrini L. Aerosol delivery in the treatment of lung cancer. Curr Cancer Drug Targets. 2015;15(7):604-612. doi: 10.2174/1568009615666150602143751. DOI: https://doi.org/10.2174/1568009615666150602143751
Wauthoz N, Rosière R, Amighi K. Inhaled cytotoxic chemotherapy: clinical challenges, recent developments, and future prospects. Expert Opin Drug Deliv. 2021;18(3):333-354. doi: 10.1080/17425247.2021.1829590. DOI: https://doi.org/10.1080/17425247.2021.1829590
Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, et al. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxidative Med Cell Longev. 2021;2021. doi: 10.1155/2021/3687700. DOI: https://doi.org/10.1155/2021/3687700
Rosière R, Van Woensel M, Mathieu V, Langer I, Mathivet T, Vermeersch M, et al. Development and evaluation of well-tolerated and tumor-penetrating polymeric micelle-based dry powders for inhaled anti-cancer chemotherapy. Int J Pharm. 2016;501(1-2):148-159. doi: 10.1016/j.ijpharm.2016.01.073. DOI: https://doi.org/10.1016/j.ijpharm.2016.01.073
Seo SJ, Das G, Shin HS, Patra JK. Silk sericin protein materials: characteristics and applications in food-sector industries. Int J Mol Sci. 2023;24(5):4951. doi: 10.3390/ijms24054951. DOI: https://doi.org/10.3390/ijms24054951
Mandal BB, Kundu S. Self-assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology. 2009;20(35):355101. doi: 10.1088/0957-4484/20/35/355101. DOI: https://doi.org/10.1088/0957-4484/20/35/355101
Nasser ST, Abdulrassol AA, Ghareeb MM. Design, preparation, and in-vitro evaluation of novel ocular antifungal nanoemulsion using posaconazole as a model drug. Int J Drug Del Technol. 2021;11(3):1058-1064. doi: 10.25258/ijddt.11.3.00.
Naji GH, Al-Gawhari FJ. Evaluation of types and concentration of bile salts impact on physical properties of nisoldipine-loaded bilosomes. Pharmacia, 2024;71:1-7. doi: 10.3897/pharmacia.71.e116917. DOI: https://doi.org/10.3897/pharmacia.71.e116917
Jasim IK, Abd Alhammid SN, Abdulrasool AA. Synthesis and evaluation of B-cyclodextrin based nanosponges of 5-Fluorouracil by using ultrasound assisted method. Iraqi J Pharm Sci. 2020;29(2):88-98. doi: 10.31351/vol29iss2pp88-98. DOI: https://doi.org/10.31351/vol29iss2pp88-98
Xu X, Wang Y, Luo X, Gao X, Gu W, Ma Y, et al. A non-invasive strategy for suppressing asthmatic airway inflammation and remodeling: Inhalation of nebulized hypoxic hUCMSC-derived extracellular vesicles. Front Immunol. 2023;14:1150971. doi: 10.3389/fimmu.2023.1150971. DOI: https://doi.org/10.3389/fimmu.2023.1150971
Alexander DJ, Collins CJ, Coombs DW, Gilkison IS, Hardy CJ, Healey G, et al. Association of Inhalation Toxicologists (AIT) working party recommendation for standard delivered dose calculation and expression in non-clinical aerosol inhalation toxicology studies with pharmaceuticals. Inhal Toxicol. 2008;20(13):1179-1189. doi: 10.1080/08958370802207318. DOI: https://doi.org/10.1080/08958370802207318
Verco J, Johnston W, Baltezor M, Kuehl PJ, Gigliotti A, Belinsky SA, et al. Pharmacokinetic profile of inhaled submicron particle paclitaxel (NanoPac®) in a rodent model. J Aerosol Med Pulmonary Drug Del. 2019;32(2):99-109. doi: 10.1089/jamp.2018.1467. DOI: https://doi.org/10.1089/jamp.2018.1467
Tepper JS, Kuehl PJ, Cracknell S, Nikula KJ, Pei L, Blanchard JD. Symposium summary: Breathe in, breathe out, its easy: What you need to know about developing inhaled drugs. Int J Toxicol. 2016;35(4):376-392. doi: 10.1177/1091581815624080. DOI: https://doi.org/10.1177/1091581815624080
Al-khfajy SW, Abdulrazzaq MH, Al-Mashhadani Z. Synergistic effects of 2-deoxy-D-glucose and cinnamic acid with erlotinib on NSCLC cell line. Iraqi J Pharm Sci. 2023;32(Suppl.):136-144. doi: 10.31351/vol32issSuppl.pp136-144. DOI: https://doi.org/10.31351/vol32issSuppl.pp136-144
Jiménez-López J, Bravo-Caparrós I, Cabeza L, Nieto FR, Ortiz R, Perazzoli G, et al. Paclitaxel antitumor effect improvement in lung cancer and prevention of the painful neuropathy using large pegylated cationic liposomes. Biomed Pharmacother. 2021;133:111059. doi: 10.1016/j.biopha.2020.111059. DOI: https://doi.org/10.1016/j.biopha.2020.111059
Akram S, Al-Shammari AM, Sahib HB, Jabir MS. Papaverine enhances the oncolytic effects of newcastle disease virus on breast cancer in vitro and in vivo. Int J Microbiol. 2023;2023. doi: 10.1155/2023/3324247. DOI: https://doi.org/10.1155/2023/3324247
Boe J, Dennis J, O'driscoll B, Bauer T, Carone M, Dautzenberg B, et al. European Respiratory Society Guidelines on the use of nebulizers: Guidelines prepared by a European Respiratory Society Task Force on the use of nebulizers. Eur Resp J. 2001;18(1):228-242. doi: 10.1183/09031936.01.00220001. DOI: https://doi.org/10.1183/09031936.01.00220001
Rosiere R, Van Woensel M, Gelbcke M, Mathieu V, Hecq J, Mathivet T, et al. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol Pharm. 2018;15(3):899-910. doi: 10.1021/acs.molpharmaceut.7b00846. DOI: https://doi.org/10.1021/acs.molpharmaceut.7b00846
Fareed N, Kassab HJ. A comparative study of oral diacerein and transdermal diacerein as novasomal gel in a model of MIA induced osteoarthritis in rats. Pharmacia, 2023;70.4:1363-1371. doi: 10.3897/pharmacia.70.e111097. DOI: https://doi.org/10.3897/pharmacia.70.e111097
Rezazadeh M, Emami J, Mostafavi A, Rostami M, Hassanzadeh F, Sadeghi H, et al. A rapid and sensitive HPLC method for quantitation of paclitaxel in biological samples using liquid-liquid extraction and UV detection: Application to pharmacokinetics and tissues distribution study of paclitaxel loaded targeted polymeric micelles. J Pharm Pharm Sci. 2015;18(5):647-660. doi: 10.18433/j3rp6z. DOI: https://doi.org/10.18433/J3RP6Z
Arts JH, Muijser H, Jonker D, van de Sandt JJ, Bos PM, Feron VJ. Inhalation toxicity studies: OECD guidelines in relation to REACH and scientific developments. Exp Toxicol Pathol. 2008;60(2-3):125-133. doi: 10.1016/j.etp.2008.01.011. DOI: https://doi.org/10.1016/j.etp.2008.01.011
Silva RM, Anderson DS, Franzi LM, Peake JL, Edwards PC, Van Winkle LS, et al. Pulmonary effects of silver nanoparticle size, coating, and dose over time upon intratracheal instillation. Toxicol Sci. 2015;144(1):151-162. doi: 10.1093/toxsci/kfu265. DOI: https://doi.org/10.1093/toxsci/kfu265
Elbardisy B, Boraie N, Galal S. Tadalafil nanoemulsion mists for treatment of pediatric pulmonary hypertension via nebulization. Pharmaceutics. 2022;14(12):2717. doi: 10.3390/pharmaceutics14122717. DOI: https://doi.org/10.3390/pharmaceutics14122717
Srinivas A, Rao PJ, Selvam G, Murthy PB, Reddy PN. Acute inhalation toxicity of cerium oxide nanoparticles in rats. Toxicol Lett. 2011;205(2):105-115. doi: 10.1016/j.toxlet.2011.05.1027. DOI: https://doi.org/10.1016/j.toxlet.2011.05.1027
Malik B, Al-Khedairy EB. Formulation and in vitro/in vivo evaluation of silymarin solid dispersion-based topical gel for wound healing. Iraqi J Pharm Sci. 2023;32(Suppl.):42-53. doi: 10.31351/vol32issSuppl.pp42-53. DOI: https://doi.org/10.31351/vol32issSuppl.pp42-53
Taher SS, Sadeq ZA, Al-Kinani KK, Alwan ZS. Solid lipid nanoparticles as promising approach for delivery of anticancer agents. Military Med Sci Letters. 2022;91(3). doi: 10.31482/mmsl.2021.042. DOI: https://doi.org/10.31482/mmsl.2021.042
Stage TB, Bergmann TK, Kroetz DL. Clinical pharmacokinetics of paclitaxel monotherapy: an updated literature review. Clin Pharmacokinet. 2018;57(1):7-19. doi: 10.1007/s40262-017-0563-z. DOI: https://doi.org/10.1007/s40262-017-0563-z
Esim O, Bakirhan NK, Sarper M, Savaser A, Ozkan SA, Ozkan Y. Influence of emulsifiers on the formation and in vitro anticancer activity of epirubicin loaded PLGA nanoparticles. J Drug Del Sci Technol. 2020;60:102027. doi: 10.1016/j.jddst.2020.102027. DOI: https://doi.org/10.1016/j.jddst.2020.102027
Egla M, Hammid S. Design zolmitriptan liquisolid orodispersible tablets and their in vitro evaluation. J Chem Pharm Res. 2016;8(11):232-242. doi: 10.22159/ijpps.2017v9i1.15656. DOI: https://doi.org/10.22159/ijpps.2017v9i1.15656
Mohammed IA, Al-Gawhari FJ. Gold Nanoparticle: Synthesis, functionalization, enhancement, drug delivery and therapy: A review. Syst Rev Pharm. 2020;11(6). doi: 10.31838/srp.2020.6.127.
Islam N, Richard D. Inhaled micro/nanoparticulate anticancer drug formulations: an emerging targeted drug delivery strategy for lung cancers. Curr Cancer Drug Targets. 2019;19(3):162-178. doi: 10.2174/1568009618666180525083451 DOI: https://doi.org/10.2174/1568009618666180525083451
Hassanzadeh P, Arbabi E, Rostami F, Atyabi F, Dinarvand R. Aerosol delivery of ferulic acid-loaded nanostructured lipid carriers: A promising treatment approach against the respiratory disorders. Physiol Pharmacol. 2017;21(4):331-342. DOI: 10.1016/j.lfs.2017.11.046. DOI: https://doi.org/10.1016/j.lfs.2017.11.046
Elbatanony RS, Parvathaneni V, Kulkarni NS, Shukla SK, Chauhan G, Kunda NK, et al. Afatinib-loaded inhalable PLGA nanoparticles for localized therapy of non-small cell lung cancer (NSCLC)—development and in-vitro efficacy. Drug Del Transl Res. 2021;11:927-943. doi: 10.1007/s13346-020-00802-8. DOI: https://doi.org/10.1007/s13346-020-00802-8
Shah N, Chaudhari K, Dantuluri P, Murthy R, Das S. Paclitaxel-loaded PLGA nanoparticles surface modified with transferrin and Pluronic® P85, an in vitro cell line and in vivo biodistribution studies on rat model. J Drug Target. 2009;17(7):533-542. doi: 10.1080/10611860903046628. DOI: https://doi.org/10.1080/10611860903046628
Patlolla RR, Chougule M, Patel AR, Jackson T, Tata PN, Singh M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J Control Release. 2010;144(2):233-241. doi: 10.1016/j.jconrel.2010.02.006. DOI: https://doi.org/10.1016/j.jconrel.2010.02.006
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











