Early Detection of Miscarriage in Recurrent Pregnancy Loss Using a Novel Urinary Biomarker
DOI:
https://doi.org/10.54133/ajms.v9i2.2412Keywords:
Adipsin;, Antiphospholipid antibody;, Chemerin, HbA1c, Recurrent pregnancy lossAbstract
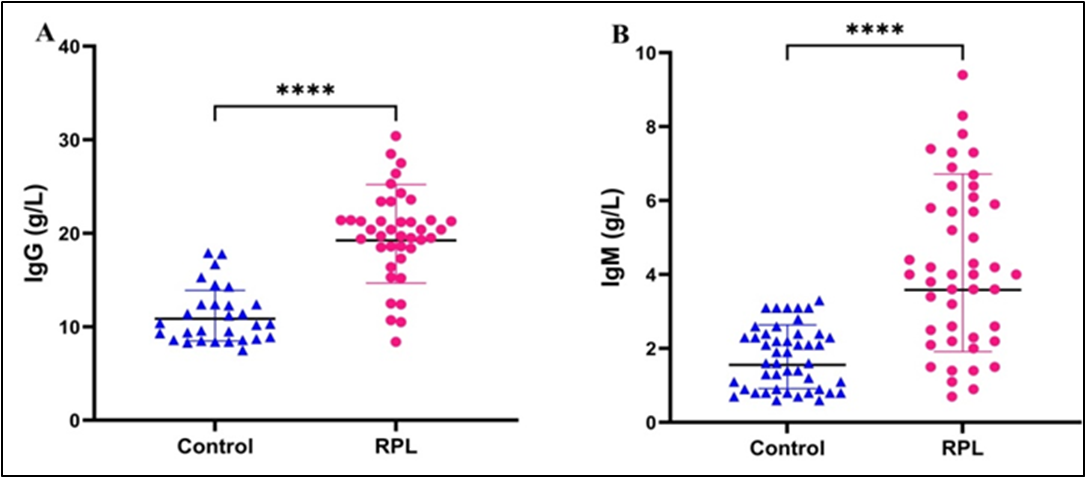
Background: Recurrent pregnancy loss (RPL) is a multifactorial reproductive disorder characterized by the occurrence of two or more consecutive pregnancy losses before 20 weeks of gestation. Objective: To assess metabolic (HbA1c, TSH), immunological antiphospholipid antibodies (aPLA) (IgM and IgG), and urinary (chemerin, adipsin) biomarkers in women with recurrent pregnancy loss and to evaluate their potential as predictors of subsequent miscarriage. Methods: In this case-control study, 92 women participated; 46 had experienced two or more consecutive pregnancy losses before 20 weeks of gestation, and 46 were healthy control women. Venous blood samples were collected to assess levels of TSH, Hb, HbA1c, and aPLA (IgG and IgM). Additionally, urine samples were collected and processed for the examination of chemerin, adipsin, and cAMP concentrations. Results: A total of ninety-two women were involved in the study. The RPL group showed significantly higher TSH, HbA1c, aPLA (IgG, IgM), and urinary chemerin, adipsin, and cAMP compared with controls. Strong correlations with RPL were observed for HbA1c (r= 0.636) and urinary adipsin (r= 0.677), with moderate correlations for TSH (r= 0.514), chemerin (r= 0.598), cAMP (r= 0.474), aPLA IgG (r= 0.460), and aPLA IgM (r= 0.578). Logistic regression identified elevated HbA1c, TSH, adipsin, chemerin, aPLA IgG/IgM, and maternal age as significant predictors of RPL. Conclusions: Metabolic (TSH, HbA1c), immunological (IgG, IgM), and urinary biomarkers (chemerin, adipsin, cAMP) are significantly associated with increased risk of recurrent pregnancy loss. These urinary markers may offer novel approaches for early diagnosis and risk prediction in RPL.
Downloads
References
Ali A, Elfituri A, Doumouchtsis SK, Zini ME, Jan H, Ganapathy R, Divakar H, et al. Managing couples with recurrent miscarriage: A narrative review and practice recommendations. Int J Gynaecol Obstet. 2024;164(2):499-503. doi: 10.1002/ijgo.14971. DOI: https://doi.org/10.1002/ijgo.14971
Turesheva A, Aimagambetova G, Ukybassova T, Marat A, Kanabekova P, Kaldygulova L, et al. Recurrent pregnancy loss etiology, risk factors, diagnosis, and management. Fresh look into a full box. J Clin Med. 2023;12(12):4074. doi: 10.3390/jcm12124074 DOI: https://doi.org/10.3390/jcm12124074
Al-Fahham AA. Etiology of antisperm antibodies in the serum of virgins. Open J Obstet Gynecol. 2018;8(3):236-245. doi: 10.1016/S0015-0282(98)00302-1. DOI: https://doi.org/10.4236/ojog.2018.83025
Sreelatha S, Nadagoudar S, Devi A. The study of maternal and fetal outcome in pregnant women with thyroid disorders. Int J Reprod Contracept Obstet Gynecol. 2017;6(8):3507-3513. doi: 10.18203/2320-1770.ijrcog20173473. DOI: https://doi.org/10.18203/2320-1770.ijrcog20173473
Lucaccioni L, Ficara M, Cenciarelli V, Berardi A, Predieri B, Iughetti L. Long term outcomes of infants born by mothers with thyroid dysfunction during pregnancy. Acta Bio Medica. 2020;92(1):e2021010. doi: 10.23750/abm.v92i1.9696.
Tańska K, Gietka-Czernel M, Glinicki P, Kozakowski J. Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front Endocrinol. 2023;13:1049665. doi: 10.3389/fendo.2022.1049665. DOI: https://doi.org/10.3389/fendo.2022.1049665
Quan X, Lan Y, Yang X. Thyroid autoimmunity and future pregnancy outcome in women of recurrent pregnancy loss: a meta-analysis. J Assist Reprod Genet. 2023;40(11):2523-2537. doi: 10.1007/s10815-023-02933-6. DOI: https://doi.org/10.1007/s10815-023-02933-6
Biondi B, Kahaly GJ, Robertson RP. Thyroid dysfunction and diabetes mellitus: two closely associated disorders. Endocr Rev. 2019;40(3):789-824. doi: 10.1210/er.2018-00163. DOI: https://doi.org/10.1210/er.2018-00163
Li YH, Marren A. Recurrent pregnancy loss. Australian J Gen Pract. 2018;47(7):432-436. doi: 10.31128/AJGP-01-18-4459. DOI: https://doi.org/10.31128/AJGP-01-18-4459
Duman G, Bayram F. Obesity and pregnancy. Turkish J Diabetes Obes. 2018;2(3):101-105. doi: 10.25048/tjdo.2018.29. DOI: https://doi.org/10.25048/tjdo.2018.29
Sugiura-Ogasawara M. Recurrent pregnancy loss and obesity. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):489-497. doi: 10.1016/j.bpobgyn.2014.12.001. DOI: https://doi.org/10.1016/j.bpobgyn.2014.12.001
Cavalcante MB, Sarno M, Peixoto AB, Araujo Junior E, Barini R. Obesity and recurrent miscarriage: A systematic review and meta‐analysis. J Obstet Gynaecol Res. 2019;45(1):30-38. doi: 10.1111/jog.13799. DOI: https://doi.org/10.1111/jog.13799
Alijotas-Reig J, Esteve-Valverde E, Anunciación-Llunell A, Marques-Soares J, Pardos-Gea J, Miró-Mur F. Pathogenesis, diagnosis and management of obstetric antiphospholipid syndrome: a comprehensive review. J Clin Med. 2022;11(3):675. doi: 10.3390/jcm11030675. DOI: https://doi.org/10.3390/jcm11030675
Chaturvedi S, McCrae KR. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. 2017;31(6):406-417. doi: 10.1016/j.blre.2017.07.006. DOI: https://doi.org/10.1016/j.blre.2017.07.006
Schreiber K, Hunt BJ. Managing antiphospholipid syndrome in pregnancy. Thrombosis Res. 2019;181:S41-S46. doi: 10.1016/S0049-3848(19)30366-4. DOI: https://doi.org/10.1016/S0049-3848(19)30366-4
Yu M, Yang Y, Huang C, Ge L, Xue L, Xiao Z, et al. Chemerin: a functional adipokine in reproductive health and diseases. Biomedicines. 2022;10(8):1910. doi: 10.3390/biomedicines10081910. DOI: https://doi.org/10.3390/biomedicines10081910
Yang X, Yao J, Wei Q, Ye J, Yin X, Quan X, et al. Role of chemerin/CMKLR1 in the maintenance of early pregnancy. Front Med. 2018;12(5):525-532. doi: 10.1007/s11684-017-0577-9. DOI: https://doi.org/10.1007/s11684-017-0577-9
Yin Y, Xie S, Xu Q, Liao L, Chen H, Zhou R. Circulating chemerin levels in preeclampsia: a systematic review and meta-analysis. Lipid Health Dis. 2023;22(1):179. doi: 10.1186/s12944-023-01941-w. DOI: https://doi.org/10.1186/s12944-023-01941-w
Xu Y, Ma M, Ippolito GC, Schroeder HW, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci. 2001;98(25):14577-82. doi: 10.1073/pnas.261428398. DOI: https://doi.org/10.1073/pnas.261428398
Poveda NE, Garcés MF, Ruiz-Linares CE, Varón D, Valderrama S, Sanchez E, et al. Serum adipsin levels throughout normal pregnancy and preeclampsia. Sci Rep. 2016;6(1):20073. doi: 10.1038/srep20073. DOI: https://doi.org/10.1038/srep20073
Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol. 2013;4:71. doi: 10.3389/fendo.2013.00071. DOI: https://doi.org/10.3389/fendo.2013.00071
Metwally M, Saravelos SH, Ledger WL, Li TC. Body mass index and risk of miscarriage in women with recurrent miscarriage. Fertil Steril. 2010;94(1):290-295. doi: 10.1016/j.fertnstert.2009.03.021. DOI: https://doi.org/10.1016/j.fertnstert.2009.03.021
Wang RQ, Deng ZM, Chen GT, Dai FF, Xia LB. Obesity and recurrent spontaneous abortion: the crucial role of weight management in pregnancy. Reprod Biol Endocrinol. 2025;23(1):10. doi: 10.1186/s12958-024-01326-3. DOI: https://doi.org/10.1186/s12958-024-01326-3
Malasevskaia I, Sultana S, Hassan A, Hafez AA, Onal F, Ilgun H, et al. A 21st century epidemy-obesity: and its impact on pregnancy loss. Cureus. 2021;13(1). doi: 10.7759/cureus.12417. DOI: https://doi.org/10.7759/cureus.12417
Bashiri A, Galperin G, Zeadna A, Baumfeld Y, Wainstock T. Increased live birth rate with dydrogesterone among patients with recurrent pregnancy loss regardless of other treatments. J Clin Med. 2023;12(5):1967. doi: 10.3390/jcm12051967. DOI: https://doi.org/10.3390/jcm12051967
Mu F, Huo H, Wang C, Hu N, Wang F. A new prognostic model for recurrent pregnancy loss: assessment of thyroid and thromboelastograph parameters. Front Endocrinol. 2024;15:1415786. doi: 10.3389/fendo.2024.1415786. DOI: https://doi.org/10.3389/fendo.2024.1415786
Dimakou DB, Tamblyn J, Justin C, Coomarasamy A, Richter A. Diagnosis and management of idiopathic recurrent pregnancy loss (RPL): Current immune testing and immunomodulatory treatment practice in the United Kingdom. J Reprod Immunol. 2022;153:103662. doi: 10.1016/j.jri.2022.103662. DOI: https://doi.org/10.1016/j.jri.2022.103662
Al-Samarrai AA, Hilmi FA, Al-Allawi NA, Murad AF. Antiphospholipid antibodies in Iraqi women with recurrent mid-trimester abortions. J Lab Physicians. 2012;4(02):078-82. doi: 10.4103/0974-2727.105586. DOI: https://doi.org/10.4103/0974-2727.105586
Awad DD, Saeed IAHM, Jasim ME. The Association between antiphospholipid and coagulation in pregnant women with blood clotting. Med J Babylon. 2024;21(Suppl 2):S272-S275. doi: 10.4103/mjbl.mjbl_926_23. DOI: https://doi.org/10.4103/MJBL.MJBL_926_23
Ma Z, Chu L, Zhang Y, Lu F, Zhu Y, Wu F, et al. Is chemerin associated with gestational diabetes mellitus? a case-control study. Diabetes Metab Syndr Obes. 2023:2271-2281. doi: 10.2147/DMSO.S417632. DOI: https://doi.org/10.2147/DMSO.S417632
Zhang Q, Xiao Z, Lee CL, Duan YG, Fan X, Yeung WS, et al. The regulatory roles of chemerin-chemokine-like receptor 1 axis in placental development and vascular remodeling during early pregnancy. Front Cell Dev Biol. 2022;10:883636. doi: 10.3389/fcell.2022.883636. DOI: https://doi.org/10.3389/fcell.2022.883636
Mukherji AB, Idowu V, Zhao L, Leung LL, Shen S, Palaniappan L, et al. Chemerin levels in individuals with type 2 diabetes and a normal weight versus individuals with type 2 diabetes and obesity: An observational, cross-sectional study. Biomedicines. 2024;12(5):983. doi: 10.3390/biomedicines12050983. DOI: https://doi.org/10.3390/biomedicines12050983
Liu M, Luo X, Xu Q, Yu H, Gao L, Zhou R, et al. Adipsin of the alternative complement pathway is a potential predictor for preeclampsia in early pregnancy. Front Immunol. 2021;12:702385. doi: 10.3389/fimmu.2021.702385. DOI: https://doi.org/10.3389/fimmu.2021.702385
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











