Protective Effects of Irigenin against Cyclophosphamide-induced Nephrotoxicity in Male Rats: Comparative Study with Vitamin E
DOI:
https://doi.org/10.54133/ajms.v9i2.2316Keywords:
Cyclophosphamide, Irigenin, Nephrotoxicity, Vitamin EAbstract
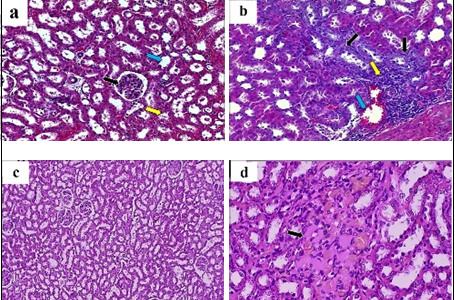
Background: Cyclophosphamide is an alkylating agent that is effective against a broad spectrum of tumors, with nephrotoxicity as a side effect. Irigenin is a natural isoflavonoid isolated from the rhizome of Belamcanda chinensis that has been reported to exert antioxidant activities. Objective: Evaluating the possible protective effects of irigenin on cyclophosphamide-induced nephrotoxicity in male rats. Methods: Fifty apparently healthy male albino rats were divided into five groups: (control, induction, irigenin, irigenin with cyclophosphamide, and vitamin E with cyclophosphamide. At the end of the experiment (day 29), all rats were sacrificed. Different parameters were evaluated, including urea and creatinine serum concentration, antioxidant markers reduced glutathione, glutathione peroxidase enzyme, and malondialdehyde level in kidney tissue homogenate, and kidney histological examination. Results: Upon cyclophosphamide administration, malondialdehyde, creatinine, and urea were increased, while their levels were reduced when irigenin was used as pretreatment. On the other hand, the reduced glutathione and glutathione peroxidase enzyme showed a reverse behavior. Additionally, the histological examination confirmed the nephroprotective effect of irigenin. Conclusions: Irigenin has a protective effect against renal damage induced by cyclophosphamide by amelioration of biochemical markers and oxidative stress parameters.
Downloads
References
Sharma V, Singh TG. Drug induced nephrotoxicity-A mechanistic approach. Mol Biol Rep. 2023;50(8):6975-6986. doi: 10.1007/s11033-023-08573-4. DOI: https://doi.org/10.1007/s11033-023-08573-4
Barnett LM, Cummings BS. Nephrotoxicity and renal pathophysiology: a contemporary perspective. Toxicol Sci. 2018;164(2):379-390. doi: 10.1093/toxsci/kfy159. DOI: https://doi.org/10.1093/toxsci/kfy159
Santos ML, de Brito BB, da Silva FA, dos Santos Botelho AC, de Melo FF. Nephrotoxicity in cancer treatment: An overview. World J Clin Oncol. 2020;11(4):190. doi: 10.5306/wjco.v11.i4.190.
Lameire N. Nephrotoxicity of recent anti-cancer agents. Clin Kidney J. 2014;7(1):11-22. doi: 10.1093/ckj/sft135. DOI: https://doi.org/10.1093/ckj/sft135
Gunes S, Sahinturk V, Uslu S, Ayhanci A, Kacar S, Uyar R. Protective effects of selenium on cyclophosphamide-induced oxidative stress and kidney injury. Biol Trace Elem Res. 2018;185:116-123. doi: 10.1007/s12011-017-1231-8.
Fang X, Zhao H, Xu T, Wu H, Sheng G. Anti-inflammatory and antioxidant effects of irigenen alleviate osteoarthritis progression through Nrf2/HO-1 pathway. Pharmaceuticals. 2024;17(10):1268. doi: 10.3390/ph17101268. DOI: https://doi.org/10.3390/ph17101268
Guo F, Wang X, Liu X. Protective effects of irigenin against 1‐methyl‐4‐phenylpyridinium‐induced neurotoxicity through regulating the Keap1/Nrf2 pathway. Phytother Res. 2021;35(3):1585-1596. doi: 10.1002/ptr.6926. DOI: https://doi.org/10.1002/ptr.6926
Zhan Y, Kong S, Fan L, Jiang J. Irigenin exhibits anticancer activity against human colon cancer cells via autophagy, inhibition of cell migration and invasion, and targeting of ERK/MAPK signal pathway. Tropic J Pharm Res. 2021;20(7):1357-1363. doi: 10.4314/tjpr.v20i7.6. DOI: https://doi.org/10.4314/tjpr.v20i7.6
Liu D, Wang Q, Yuan W, Wang Q. Irigenin attenuates lipopolysaccharide-induced acute lung injury by inactivating the mitogen-activated protein kinase (MAPK) signaling pathway. Hum Exp Toxicol. 2023;42:09603271231155098. doi: 10.1177/09603271231155098.
Xiong Z, Liu L, Jian Z, Ma Y, Li H, Jin X, et al. Vitamin E and multiple health outcomes: an umbrella review of meta-analyses. Nutrients. 2023;15(15):3301. doi: 10.3390/nu15153301. DOI: https://doi.org/10.3390/nu15153301
Obaid AA, Alsammak MI, Fadhil MS. The effect of vitamin E on the histological structure of kidney in rats treated with cyclophosphamide. Iraqi J Vet Sci. 2022;36(2):513-517. DOI: https://doi.org/10.33899/ijvs.2021.130689.1865
Neuzil J, Weber T, Terman A, Weber C, Brunk UT. Vitamin E analogues as inducers of apoptosis: implications for their potential antineoplastic role. Redox Rep. 2001;6(3):143-151. doi: 10.1179/135100001101536247. DOI: https://doi.org/10.1179/135100001101536247
Noguchi N, Niki E. Vitamin E nomenclature. Is RRR-α-tocopherol the only vitamin E? Free Radic Biol Med. 2024. doi: 10.1016/j.freeradbiomed.2024.05.027. DOI: https://doi.org/10.1016/j.freeradbiomed.2024.05.027
Vitamin E - Page vii. Available at: https://books.google.iq
Ilçe F, Gök G, Pandir D. Acute effects of lipopolysaccharide (LPS) in kidney of rats and preventive role of vitamin E and sodium selenite. Hum Exp Toxicol. 2019;38(5):547-560. doi: 10.1177/0960327118817106. DOI: https://doi.org/10.1177/0960327118817106
Birringer M, Lorkowski S. Vitamin E: Regulatory role of metabolites. IUBMB life. 2019;71(4):479-486. doi: 10.1002/iub.1988. DOI: https://doi.org/10.1002/iub.1988
Amanpour P, Khodarahmi P, Salehipour M. Protective effects of vitamin E on cadmium-induced apoptosis in rat testes. Arch Pharmacol. 2020;393(3):349-358. doi: 10.1007/s00210-019-01736-w. DOI: https://doi.org/10.1007/s00210-019-01736-w
Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR. Behavioral effects of vehicles: DMSO, ethanol, Tween-20, Tween-80, and emulphor-620. Pharmacol Biochem Behav. 1995;50(4):521-526. doi: 10.1016/0091-3057(94)00331-9. DOI: https://doi.org/10.1016/0091-3057(94)00331-9
Gunes S, Sahinturk V, Uslu S, Ayhanci A, Kacar S, Uyar R. Protective effects of selenium on cyclophosphamide-induced oxidative stress and kidney injury. Biol Trace Elem Res. 2018;185:116-123. doi: 10.1007/s12011-017-1231-8. DOI: https://doi.org/10.1007/s12011-017-1231-8
Dobrek L, Baranowska A, Skowron B, Thor P. Biochemical and histological evaluation of kidney function in rats after a single administration of cyclophosphamide and ifosfamide. J Nephrol Kidney Dis. 2017;1(1):1002-1008. doi: 10.36876/smjnkd.1002. DOI: https://doi.org/10.36876/smjnkd.1002
Sinanoglu O, Yener AN, Ekici S, Midi A, Aksungar FB. The protective effects of spirulina in cyclophosphamide induced nephrotoxicity and urotoxicity in rats. Urology. 2012;80(6):1392. doi: 10.1016/j.urology.2012.06.053. DOI: https://doi.org/10.1016/j.urology.2012.06.053
Zhang G, Liao Y, Yang H, Tao J, Ma L, Zuo X. Irigenin reduces the expression of caspase-3 and matrix metalloproteinases, thus suppressing apoptosis and extracellular matrix degradation in TNF-α-stimulated nucleus pulposus cells. Chem Biol Interact. 2021;349:109681. doi: 10.1016/j.cbi.2021.109681. DOI: https://doi.org/10.1016/j.cbi.2021.109681
Liu D, Wang Q, Yuan W, Wang Q. Irigenin attenuates lipopolysaccharide-induced acute lung injury by inactivating the mitogen-activated protein kinase (MAPK) signaling pathway. Hum Expe Toxicol. 2023;42:09603271231155098. doi: 10.1016/j.cbi.2021.109681. DOI: https://doi.org/10.1177/09603271231155098
Bhatti GK, Bhatti JS, R Kiran R, Sandhir R. Alterations in Ca²⁺ homeostasis and oxidative damage induced by ethion in erythrocytes of Wistar rats: ameliorative effect of vitamin E. Environ Toxicol Pharmacol. 2011;31(3):378-386. doi: 10.1016/j.etap.2011.01.004. DOI: https://doi.org/10.1016/j.etap.2011.01.004
Wilcox AA, Carroll WE, Sterling RE, Davis HA, Ware AG. Use of the Berthelot reaction in the automated analysis of serum urea nitrogen. Clin Chem. 1966;12(3):151-157. doi: 10.1093/clinchem/12.3.151. DOI: https://doi.org/10.1093/clinchem/12.3.151
Liu WS, Chung YT, Yang CY, Lin CC, Tsai KH, Yang WC, et al. Serum creatinine determined by Jaffe, enzymatic method, and isotope dilution‐liquid chromatography‐mass spectrometry in patients under hemodialysis. J Clin Lab Anal. 2012;26(3):206-214. doi: 10.1002/jcla.21495. DOI: https://doi.org/10.1002/jcla.21495
https://file.elabscience.com/Manual/elisa_kits/E-EL-0026-Elabscience.pdf
https://file.elabscience.com/Manual/elisa_kits/E-EL-0060-Elabscience.pdf
https://file.elabscience.com/Manual/elisa_kits/E-EL-R2491-Elabscience.pdf
ALHaithloul HA, Alotaibi MF, Bin-Jumah M, Elgebaly H, Mahmoud AM. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed Pharmacother. 2019;111:676-685. doi: 10.1016/j.biopha.2018.12.112 DOI: https://doi.org/10.1016/j.biopha.2018.12.112
Sadeghi A, Kalantar M, Molavinia S, Houshmand G, Bahadoram M, Esmaeilizadeh M, et al. Ameliorative effects of hydroalcoholic extract of Lavandula officinalis L. on cyclophosphamide-induced nephrotoxicity in mice. J Nephropathol. 2017;6(4):324-332. doi: 10.15171/jnp.2017.52. DOI: https://doi.org/10.15171/jnp.2017.52
Rehman MU, Tahir M, Ali F, Qamar W, Lateef A, Khan R, et al. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: the protective effect of Ellagic acid. Mol Cell Biochem. 2012;365:119-127. doi: 10.15171/jnp.2017.52. DOI: https://doi.org/10.1007/s11010-012-1250-x
Lopes FF, Faverzani JL, Hammerschmidt T, Delgado CA, De Oliveira JF, Wajner M, et al. Evaluation of oxidative damage to biomolecules and inflammation in patients with urea cycle disorders. Arch Biochem Biophys. 202;736:109526. doi: 10.1016/j.abb.2023.109526 DOI: https://doi.org/10.1016/j.abb.2023.109526
Cuce G, Çetinkaya S, Koc T, Esen HH, Limandal C, Balcı T, et al. Chemoprotective effect of vitamin E in cyclophosphamide-induced hepatotoxicity in rats. Chem Biol Interact. 2015;232:7-11. doi: 10.1016/j.cbi.2015.02.016. DOI: https://doi.org/10.1016/j.cbi.2015.02.016
Harahap Y, Nurahman F, Purwanto DJ, Yanuar A. The correlation between the level of 3-hydroxypropyl mercapturic acid, CYP2B6 polymorphisms, and hematuria occurrences after cyclophosphamide administration and its bioanalytical methods: A systematic review. Heliyon. 2021;7(10):e08126. doi: 10.1016/j.heliyon.2021.e08126. DOI: https://doi.org/10.1016/j.heliyon.2021.e08126
Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52(1):7-25. doi: 10.1002/mnfr.200700412. DOI: https://doi.org/10.1002/mnfr.200700412
Senthilkumar S, Yogeeta SK, Subashini R, Devaki T. Attenuation of cyclophosphamide induced toxicity by squalene in experimental rats. Chem Biol Interact. 2006;160:252–260. doi: 10.1002/mnfr.200700412. DOI: https://doi.org/10.1016/j.cbi.2006.02.004
Santos MLC, de Brito BB, da Silva FAF, Botelho ACDS, de Melo FF. Nephrotoxicity in cancer treatment, An overview. World J Clin Oncol. 2020;11:190–204. doi: 10.5306/wjco.v11.i4.190. DOI: https://doi.org/10.5306/wjco.v11.i4.190
Ijaz MU, Mustafa S, Batool R, Naz H, Ahmed H, Anwar H. Ameliorative effect of herbacetin against cyclophosphamide-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Hum Exp Toxicol. 2022;41:09603271221132140. doi: 10.1177/09603271221132140. DOI: https://doi.org/10.1177/09603271221132140
Guo L, Zheng X, Wang E, Jia X, Wang G, Wen J. Irigenin treatment alleviates doxorubicin (DOX)-induced cardiotoxicity by suppressing apoptosis, inflammation and oxidative stress via the increase of miR-425. Biomed Pharmacother. 2020;125:109784. doi: 10.1016/j.biopha.2019.109784. DOI: https://doi.org/10.1016/j.biopha.2019.109784
Xin RH, Zheng JF, Cheng L, Peng WJ, Luo YJ. Belamcanda chinensis (L.) Dc: Ethnopharmacology, phytochemistryand pharmacology of an important traditional Chinese medicine. Afr J Trad Complement Altern Med. 2015;12(6):39-70. doi: 10.4314/ajtcam.v12i6.6. DOI: https://doi.org/10.21010/ajtcam.v12i6.6
Estakhri R, Hajipour B, Majidi H, Soleimani H. Vitamin E ameliorates cyclophosphamide induced nephrotoxicity. Life Sci J. 2013;10(6s):308-313.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Al-Rafidain Journal of Medical Sciences ( ISSN 2789-3219 )

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Published by Al-Rafidain University College. This is an open access journal issued under the CC BY-NC-SA 4.0 license (https://creativecommons.org/licenses/by-nc-sa/4.0/).











